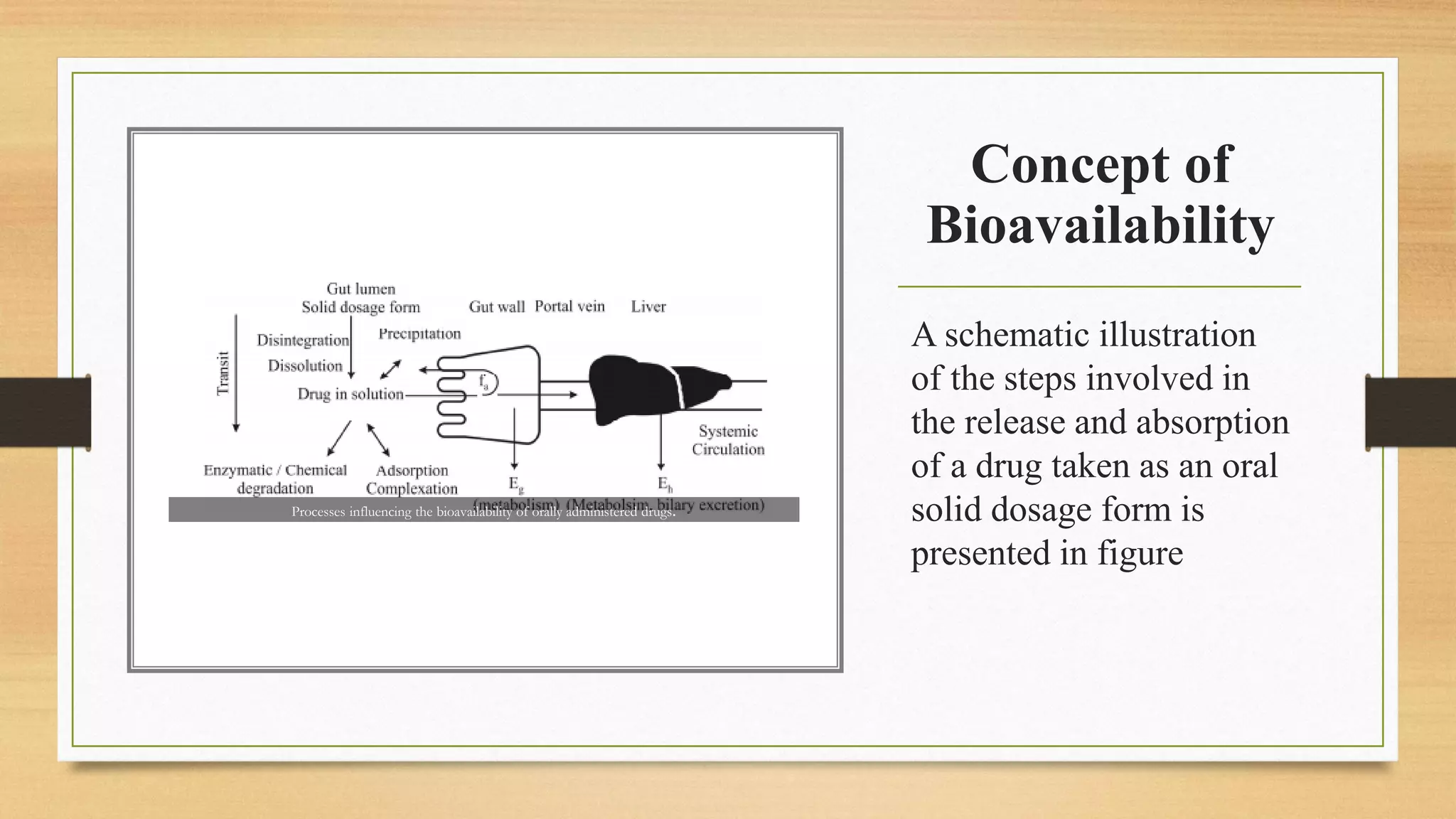
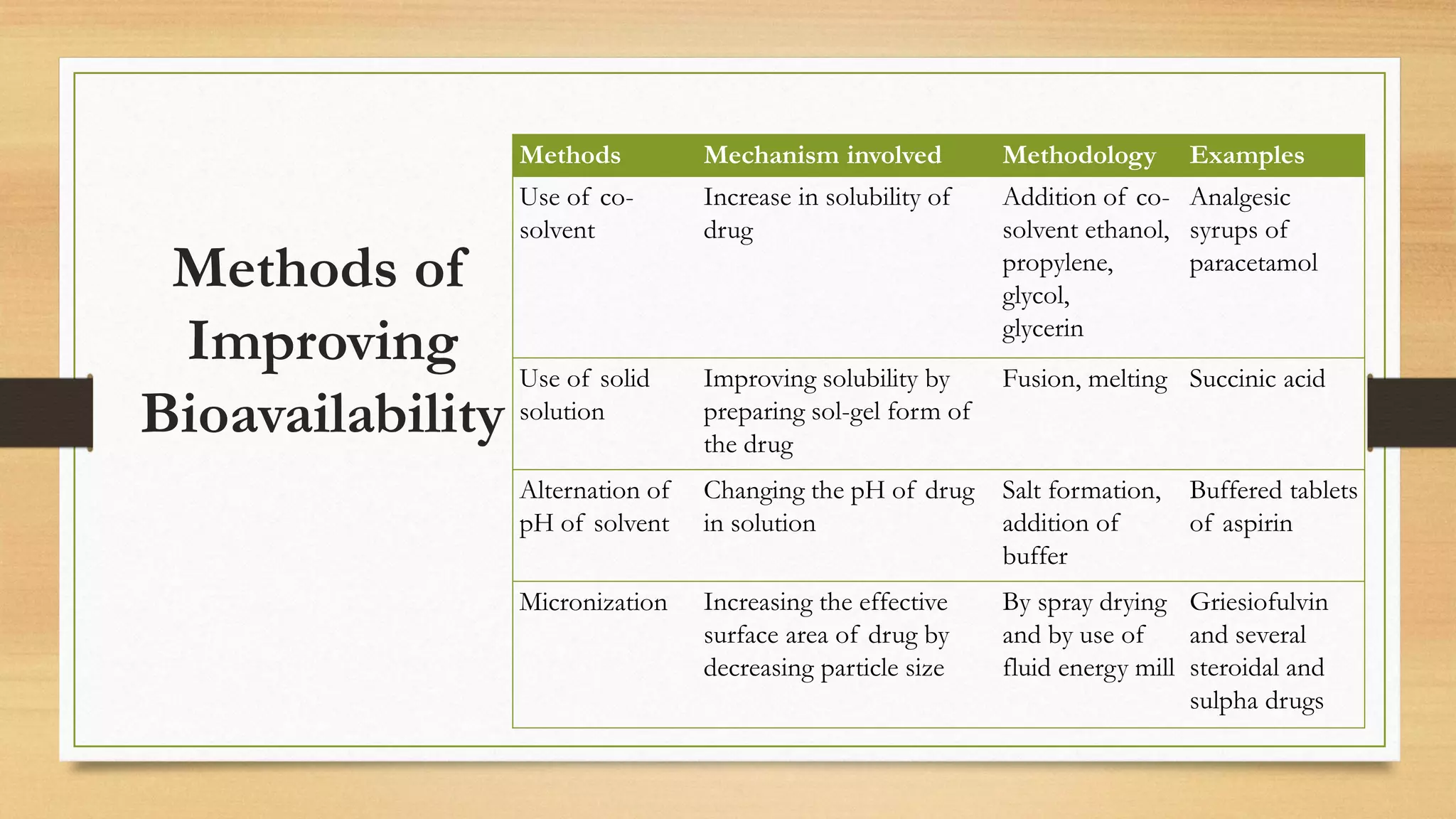
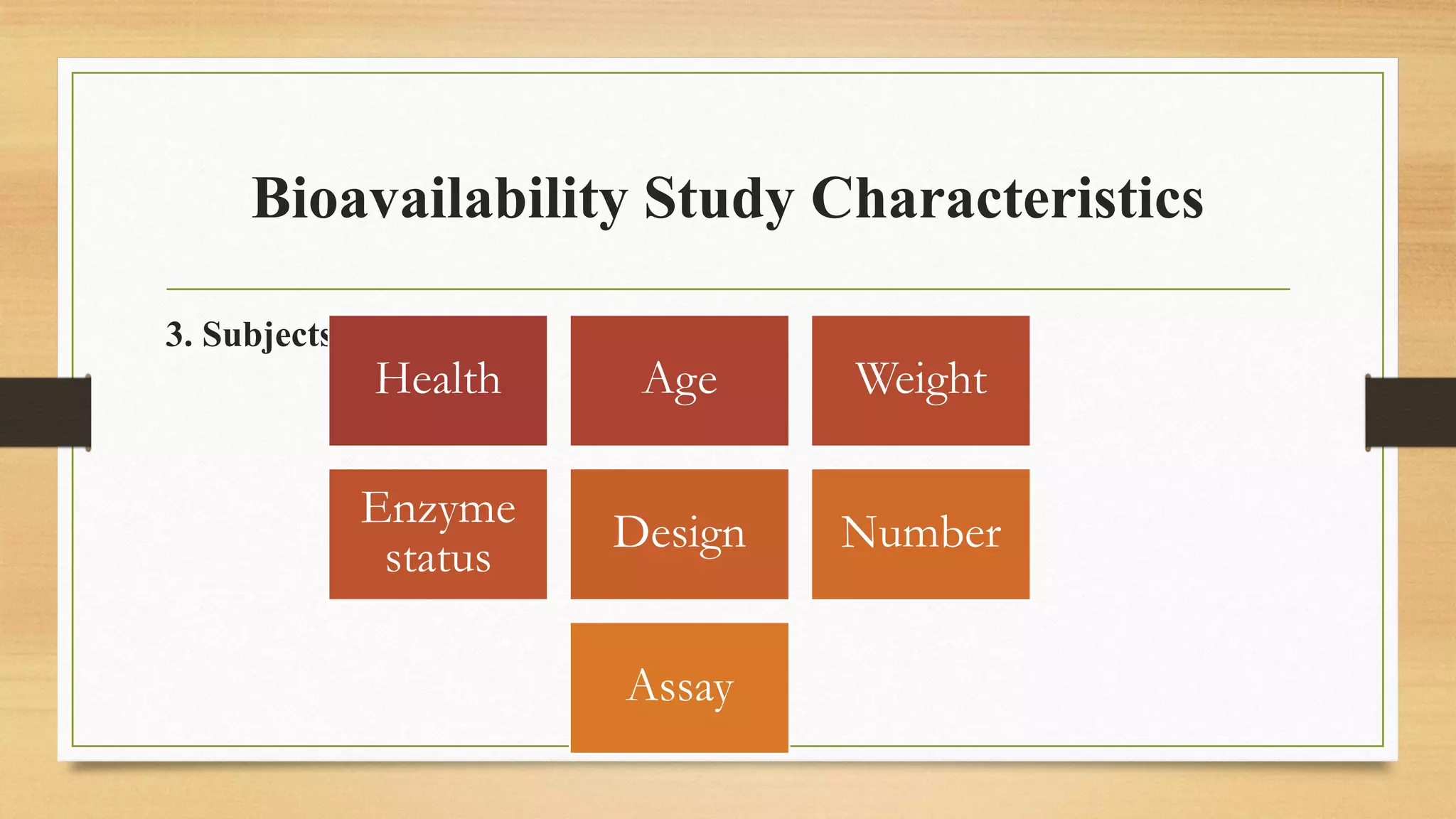
The document discusses methods to improve the bioavailability of orally administered drugs, categorizing them into pharmaceutical, pharmacokinetic, and biological approaches. It outlines various techniques, such as using co-solvents, solid solutions, and micronization, to enhance drug solubility and absorption. Additionally, it details the characteristics and considerations for conducting bioavailability studies, including drug substances, products, and the subjects involved.