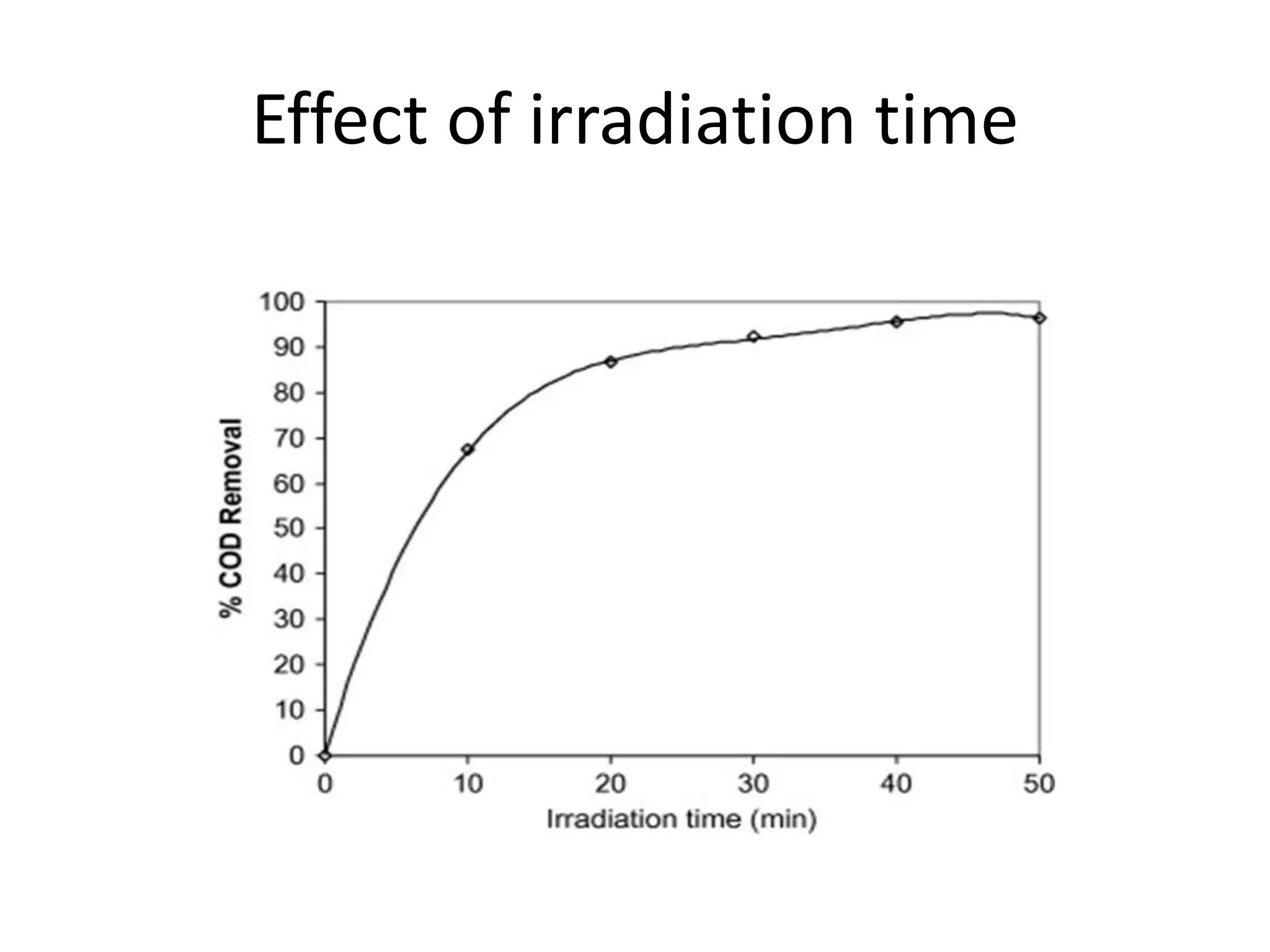
Wastewater management involves treating various sources of water pollution using advanced oxidation processes like photo-Fenton oxidation. Photo-Fenton oxidation uses UV light, hydrogen peroxide, and iron ions to produce hydroxyl radicals that effectively eliminate organic pollutants through oxidation. The process parameters that affect photo-Fenton oxidation include pH, hydrogen peroxide dose, irradiation time, and initial iron ion concentration. Photo-Fenton oxidation shows potential for treating industrial wastewater for reuse in fertilizer production after further treatment.