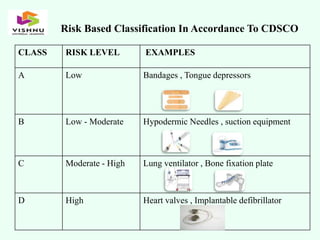
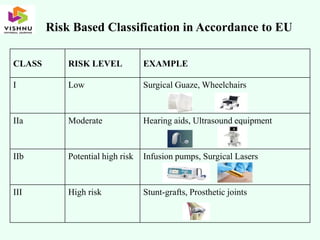
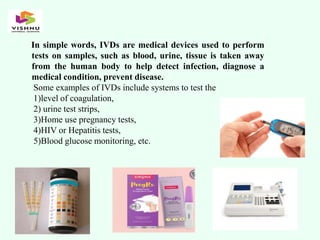
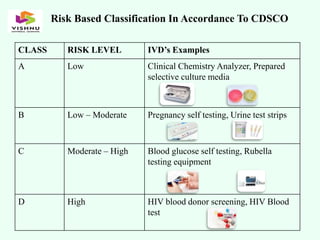
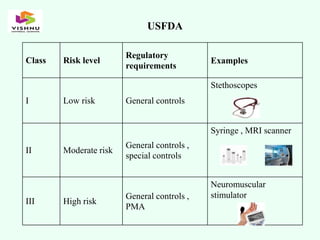
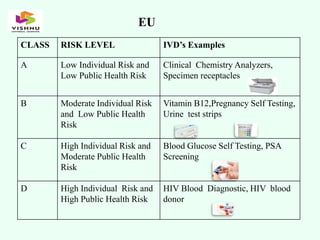
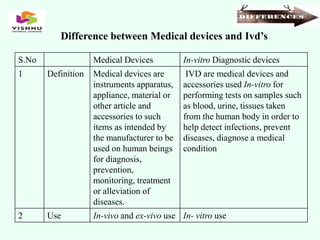
The document outlines definitions and classifications of medical devices and in-vitro diagnostics (IVDs) as per CDSCO and US FDA regulations, detailing their intended use for diagnostic and therapeutic purposes. It provides a risk-based classification system for both medical devices and IVDs, along with essential principles that manufacturers must follow to ensure safety and effectiveness. Additionally, the document discusses combination products that integrate medical devices with drugs or biological products, highlighting various categories and examples.