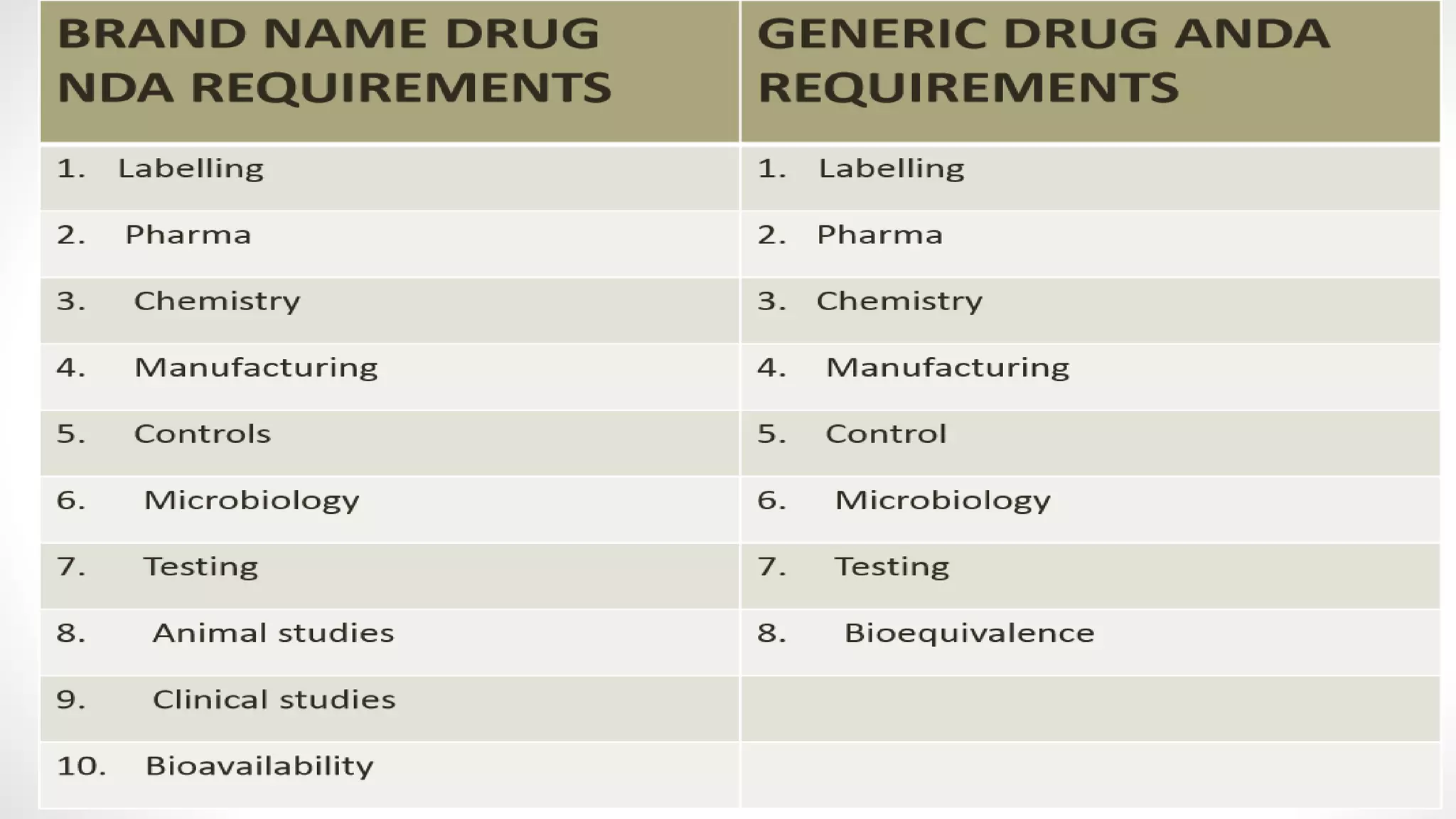
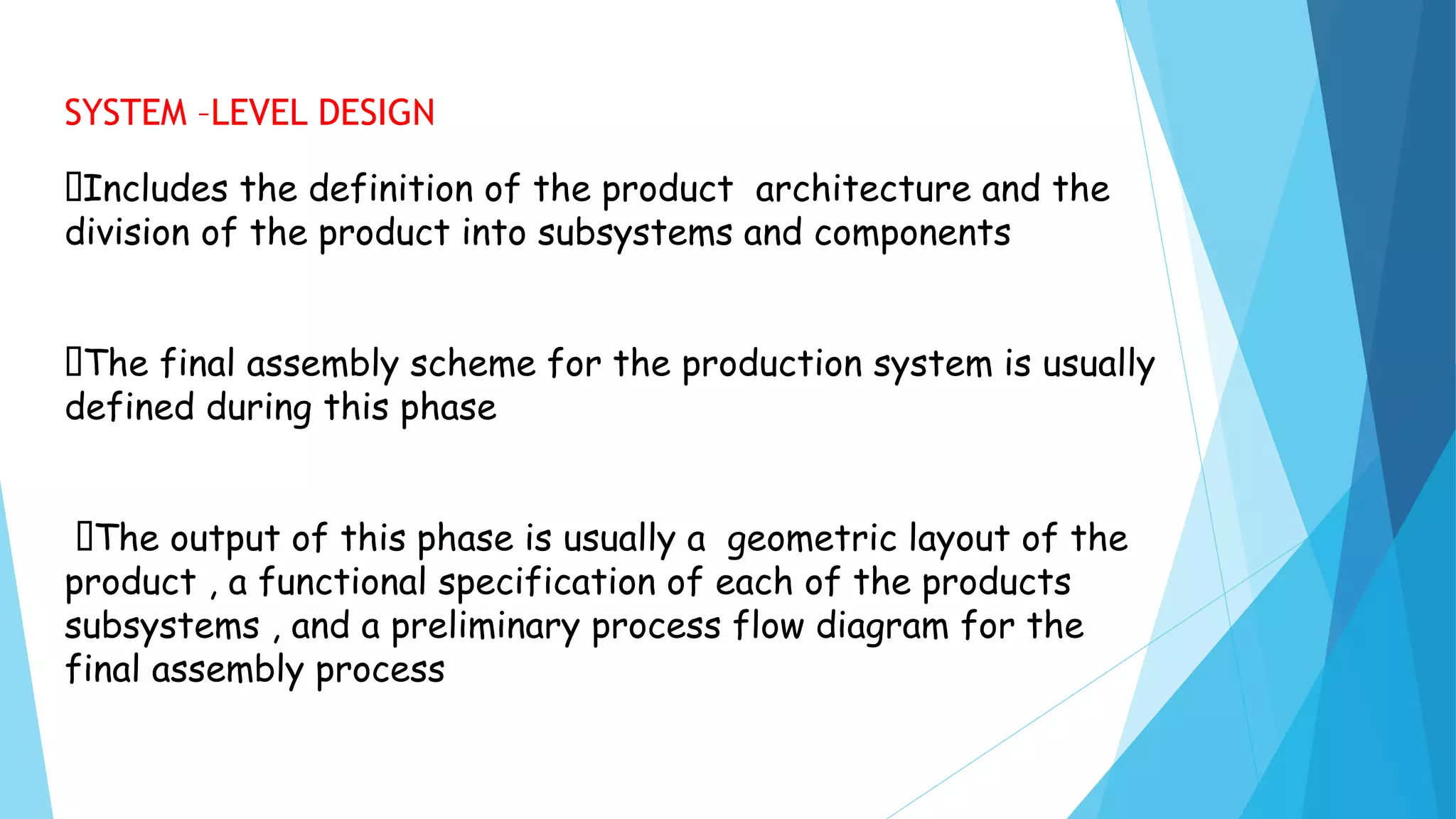
The document discusses generic drug product development. It defines a generic drug as having the same active ingredients, dosage form, strength, quality, and intended use as the branded version. Generic drugs are available after patent expiration or waiver. The generic product development process involves concept development, system-level design, detail design, testing and refinement, and production ramp-up to launch the product.