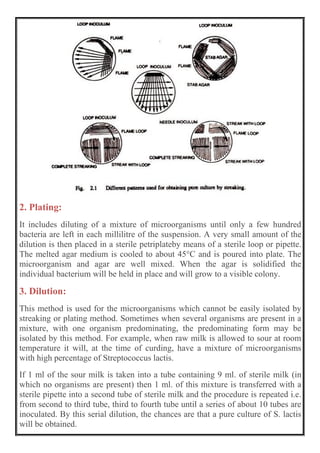
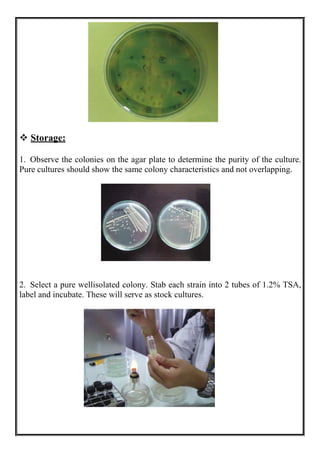
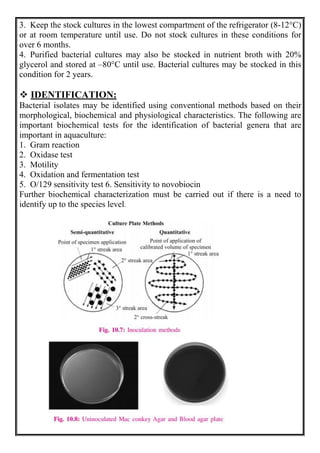
This document discusses methods for isolating bacteria from mixed cultures in order to obtain a pure culture of a single bacterial species. It describes several techniques used for isolation including streaking, plating, dilution, enrichment procedures, and single cell techniques. Streaking is the most widely used method and involves streaking bacteria across an agar plate with a sterile loop or needle to separate individual colonies. Other methods like plating, dilution, and enrichment procedures help isolate bacteria by taking advantage of differences in growth rates or nutritional requirements. Obtaining a pure culture of a single bacterial species is the first step in identifying bacteria that may cause disease.