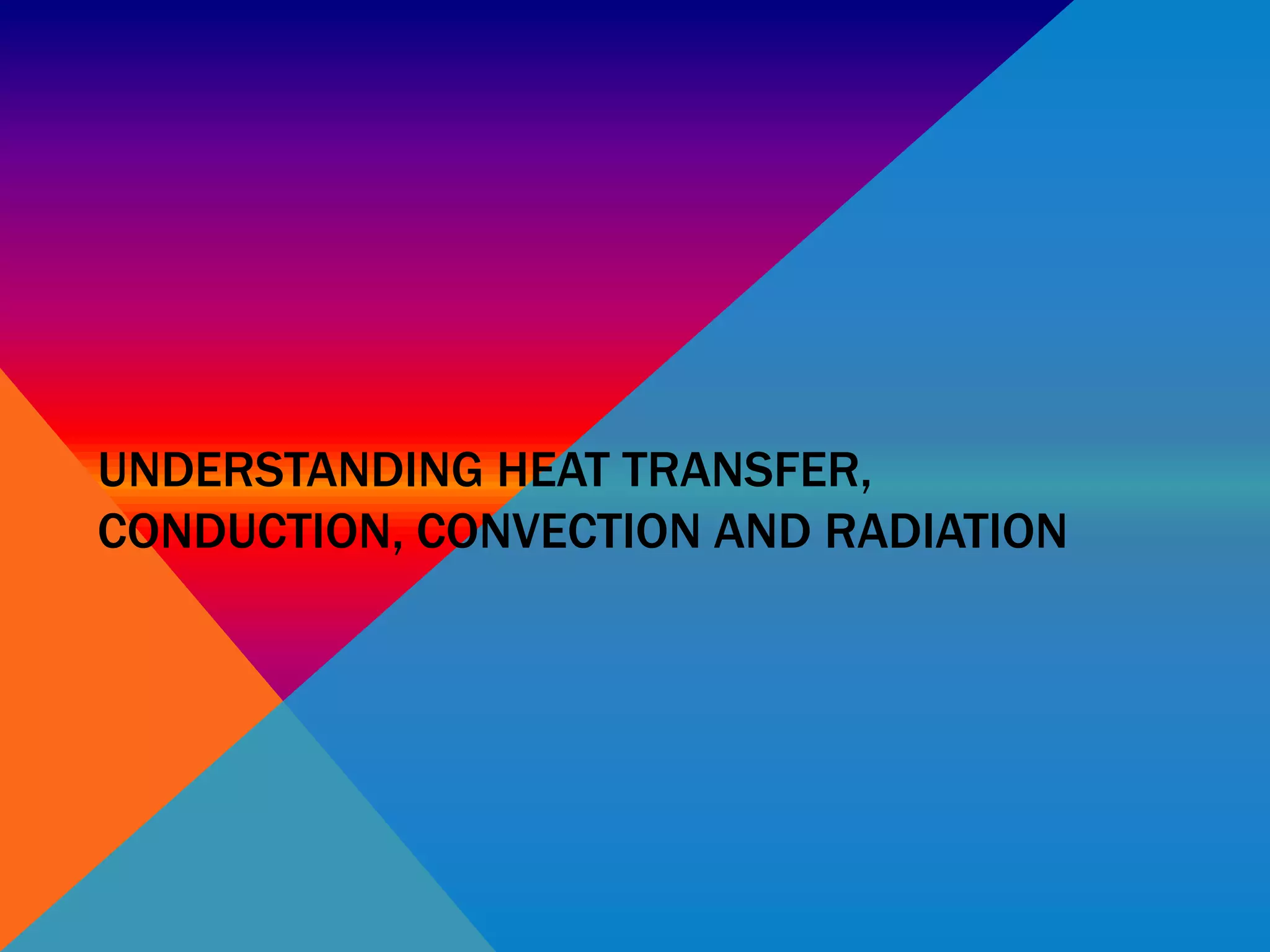
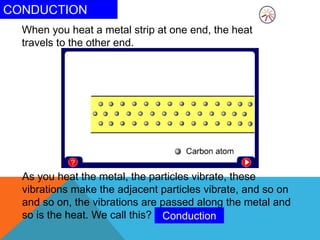
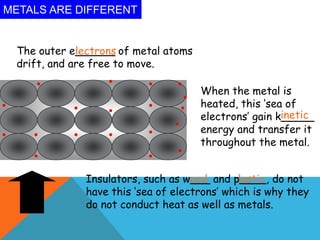
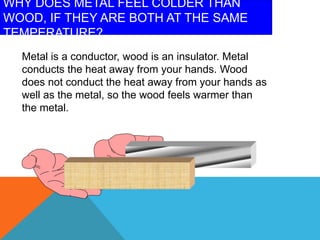
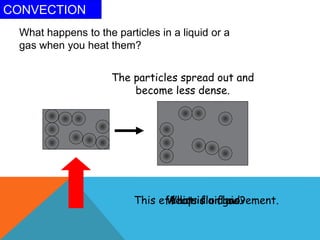
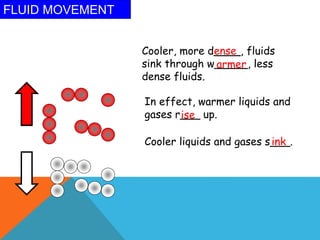
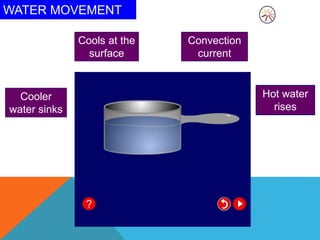
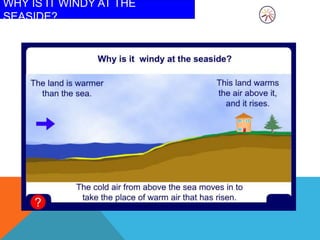
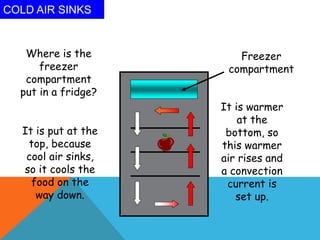
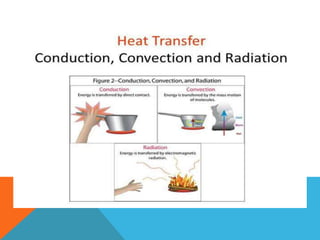
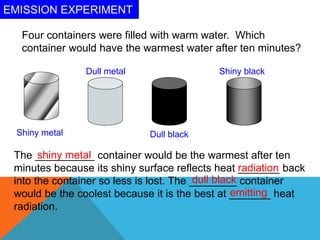
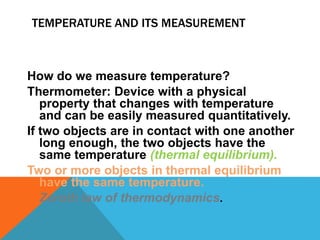
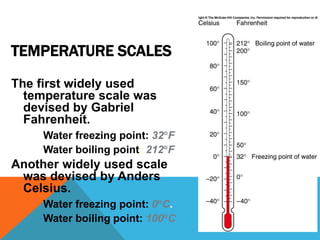
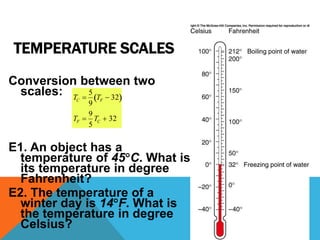
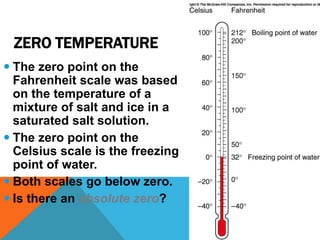
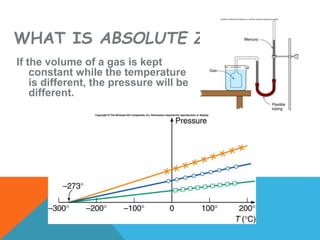
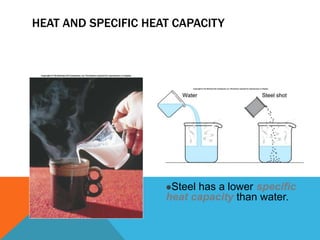
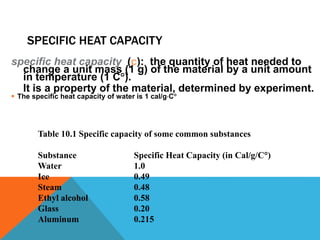
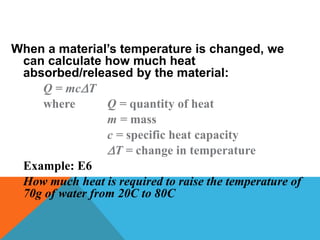
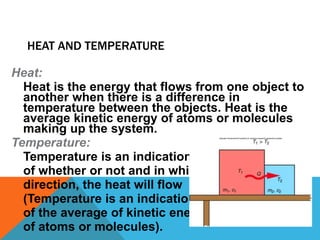
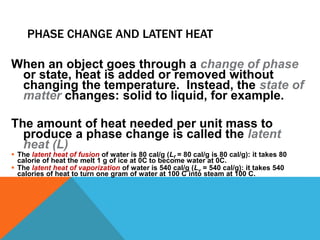
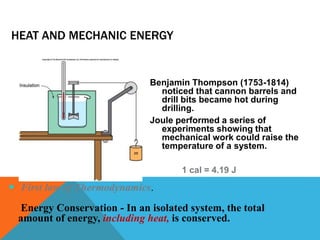
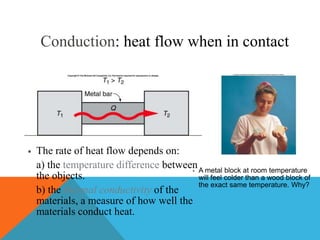
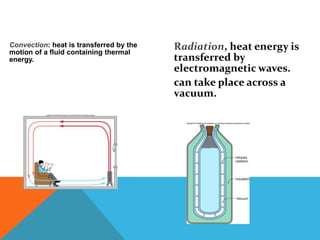
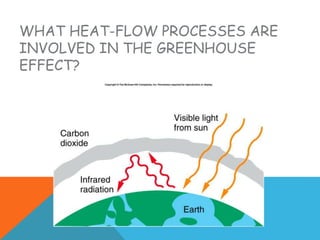
Heat can transfer between objects through three methods: conduction, convection, and radiation. Conduction involves the transfer of kinetic energy through direct contact between particles. Convection involves the transfer of heat by the circulation of fluids like gases and liquids. Radiation transfers heat through electromagnetic waves and does not require a medium.