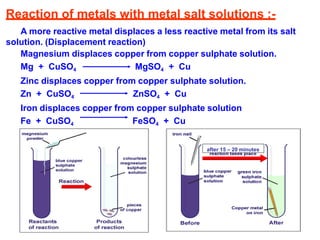
This document discusses the physical properties and reactions of metals and non-metals. Metals are solid, malleable, and good conductors of heat and electricity, while non-metals can be solid, liquid, or gas and are not malleable. Metals react differently with oxygen, water, and metal salt solutions depending on their reactivity. Most metals are found naturally as ores and must be extracted from impurities. Corrosion of metals can be prevented through various coatings and by forming alloys.