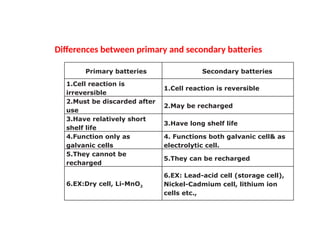
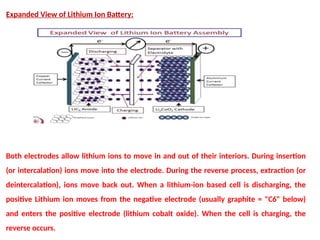
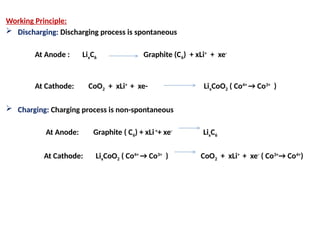
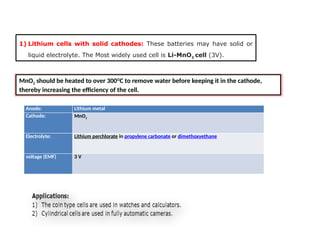
Unit 1 – Introduction to Lithium-Ion Batteries provides a foundational overview of lithium-ion battery technology, widely used in portable electronics, electric vehicles, and renewable energy systems. This unit covers the basic working principle of lithium-ion cells, key components (anode, cathode, electrolyte, and separator), and the charge-discharge mechanism. It also introduces the advantages of lithium-ion batteries such as high energy density, low self-discharge, and long cycle life, along with safety considerations and common applications. This unit lays the groundwork for understanding advanced topics like battery management systems, performance analysis, and future developments in energy storage.