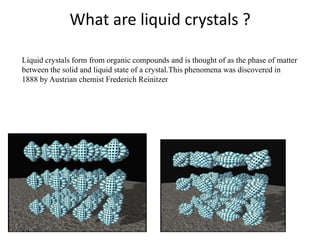
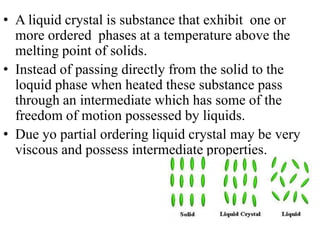
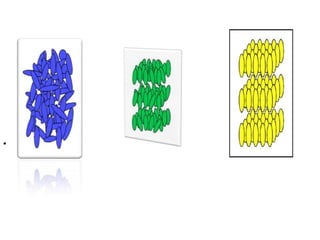
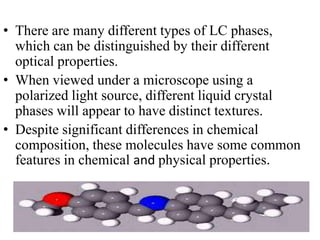
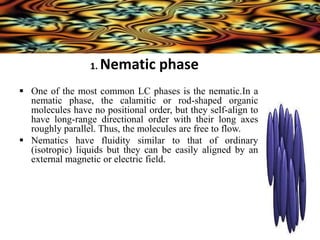
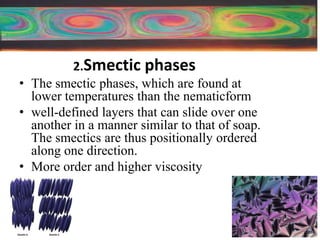
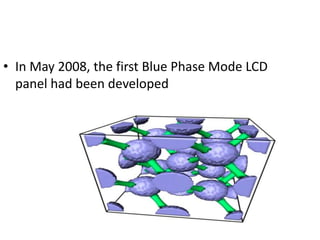
Liquid crystals are a state of matter between solid and liquid that form from organic compounds. They were discovered in 1888 by Austrian chemist Frederich Reinitzer. Liquid crystals exhibit ordered phases above melting points and have properties between solids and liquids. There are different types of liquid crystal phases including nematic, smectic, and blue phases that have distinct textures and properties. Liquid crystals find uses in devices like flat screen displays, watches, and thermometers.