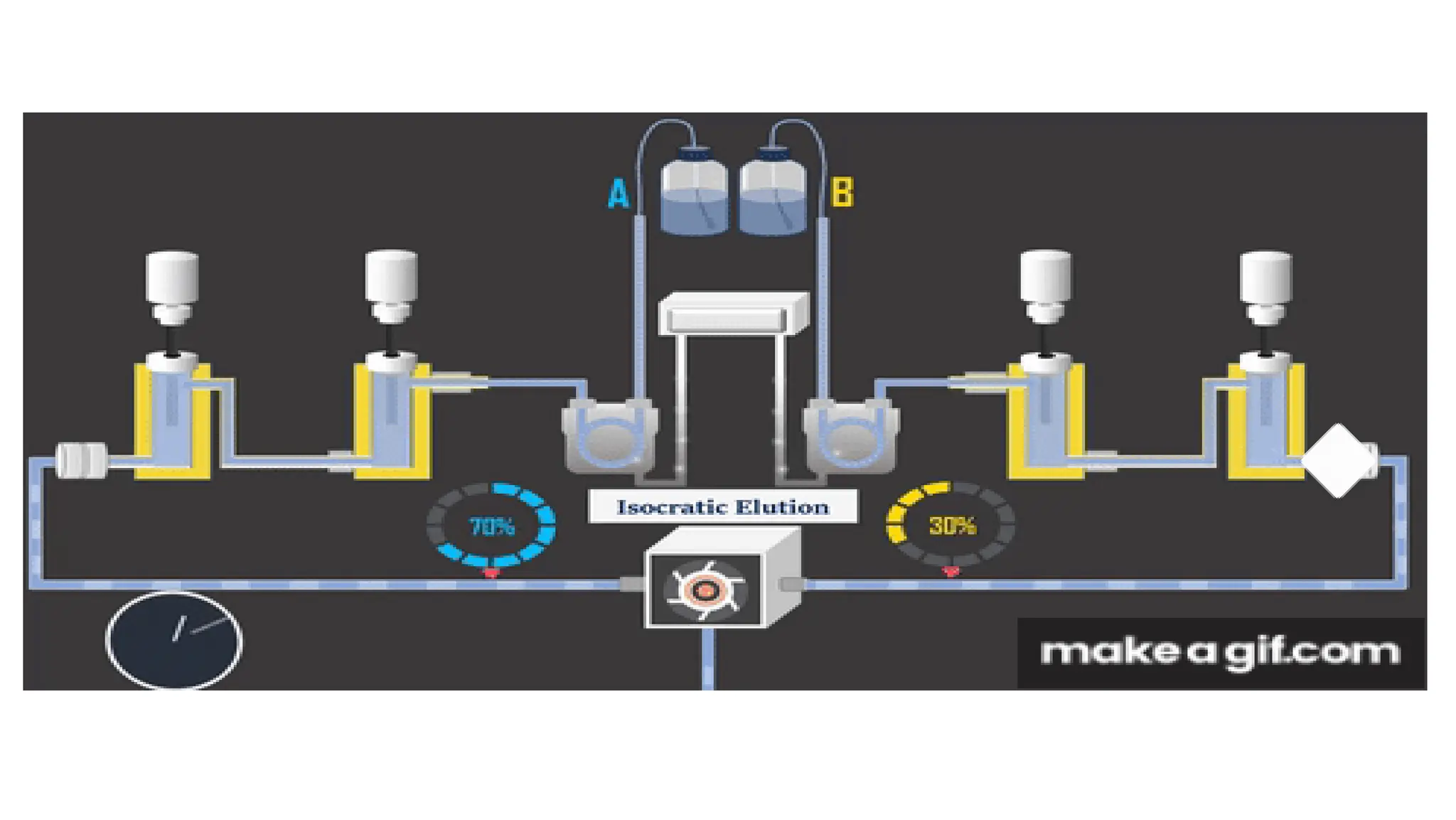
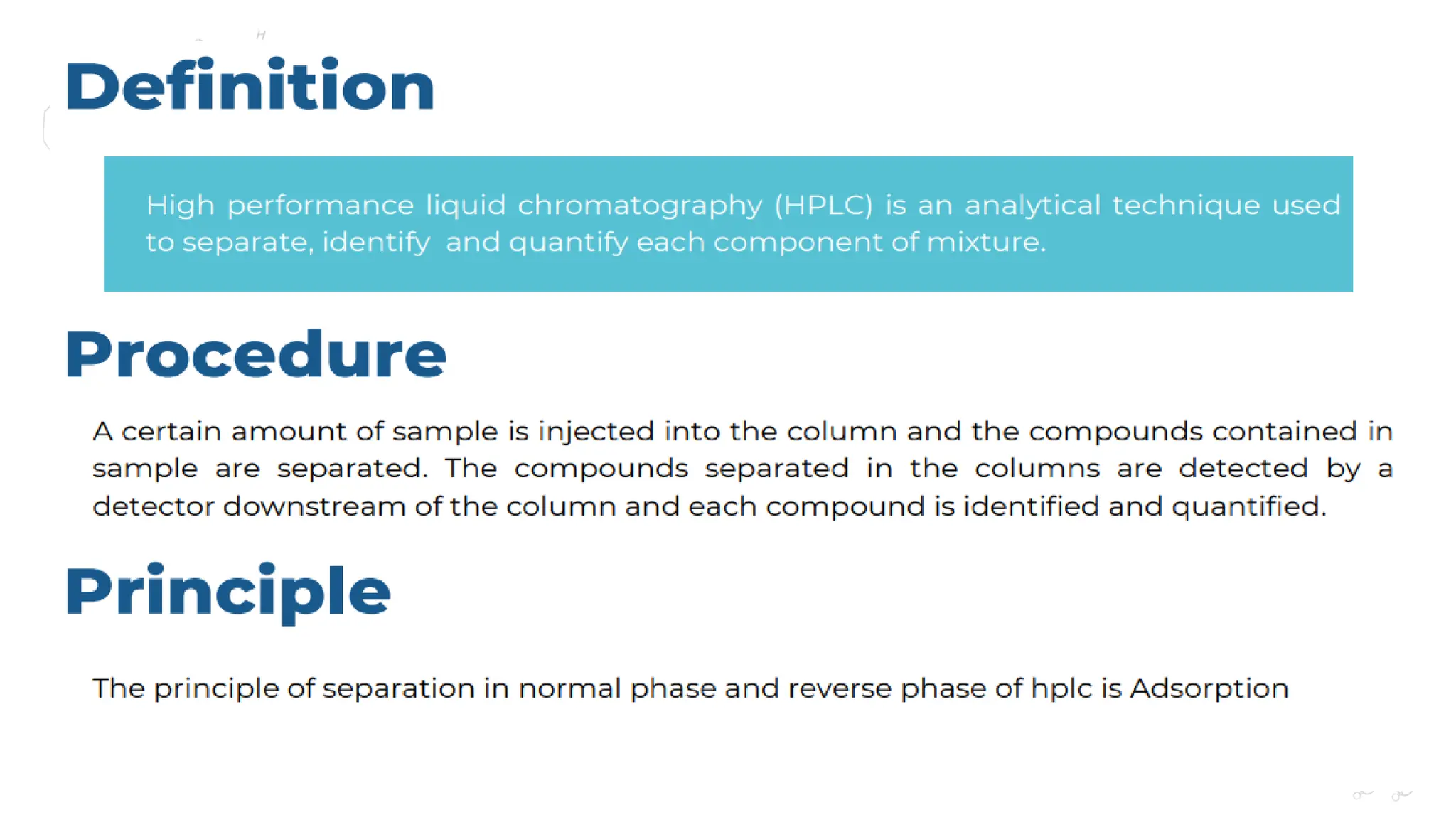
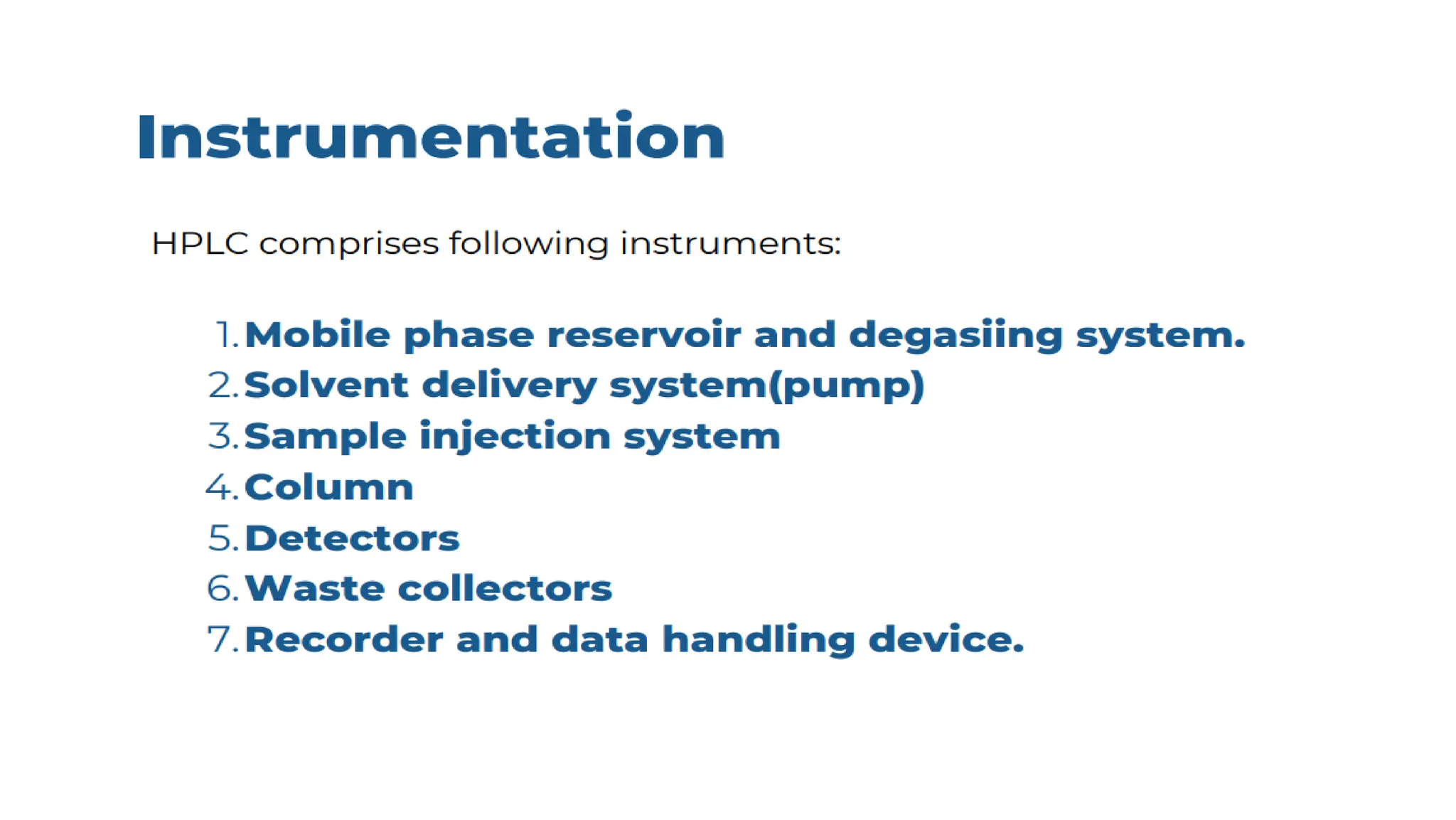
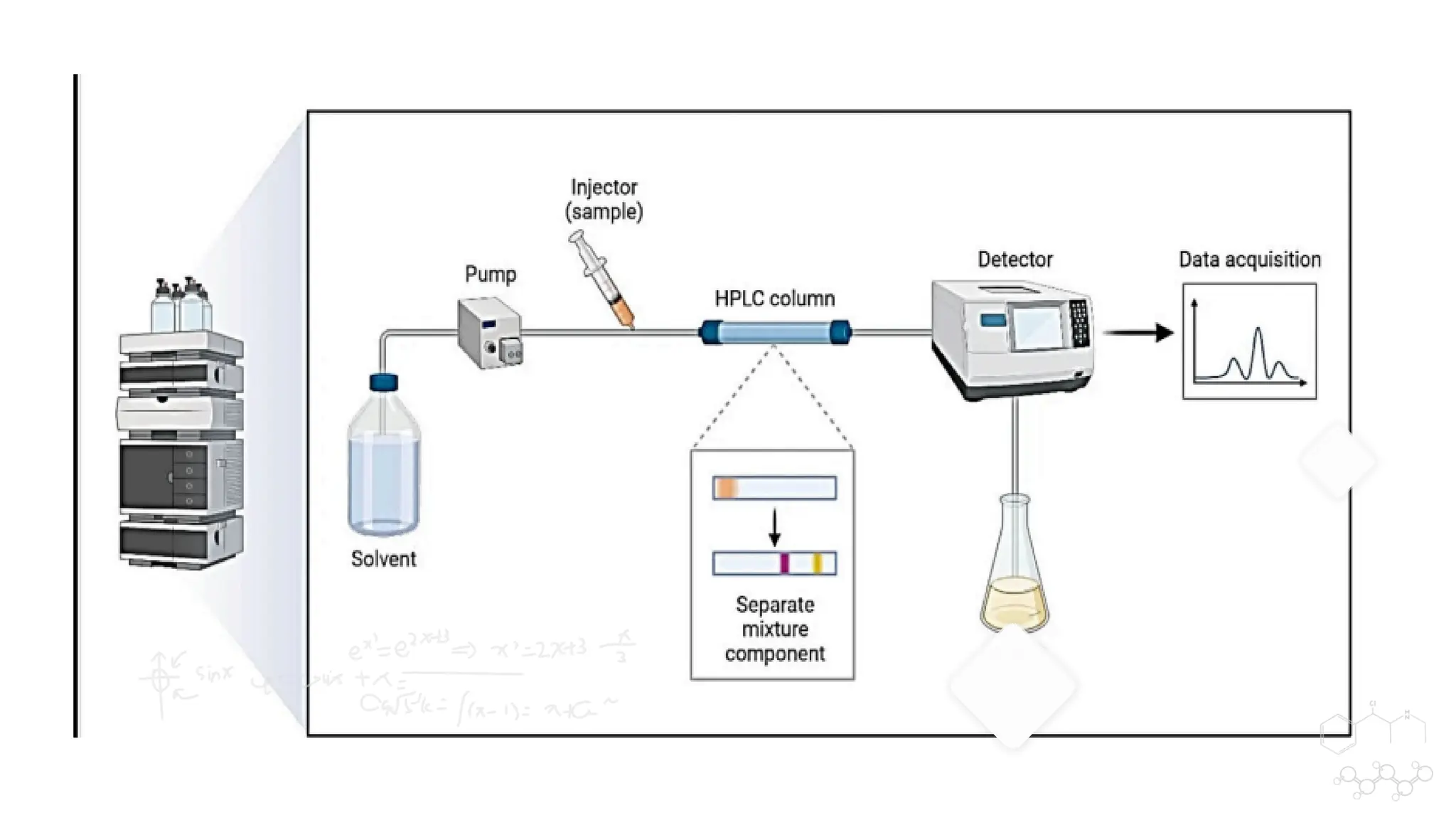
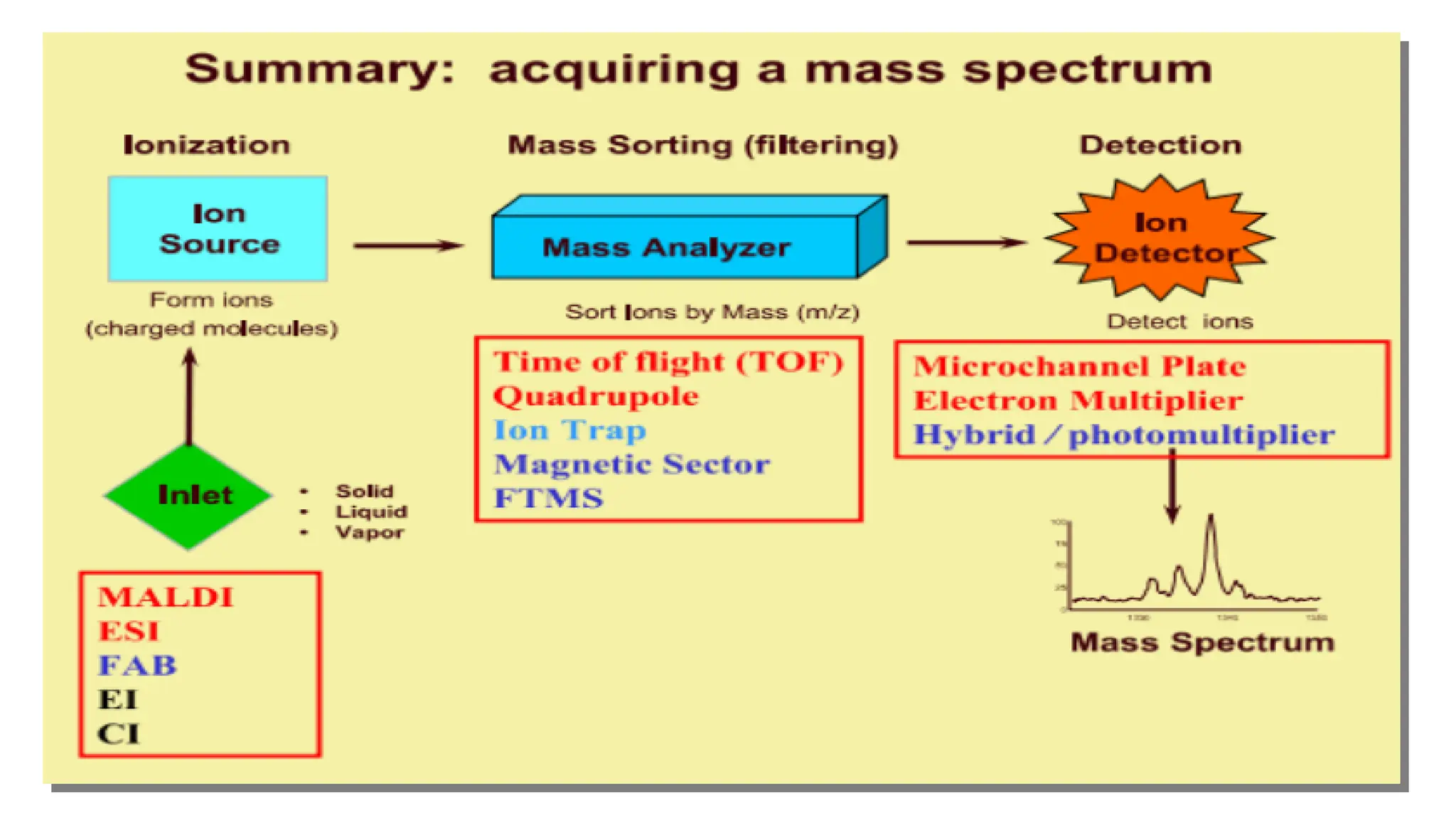
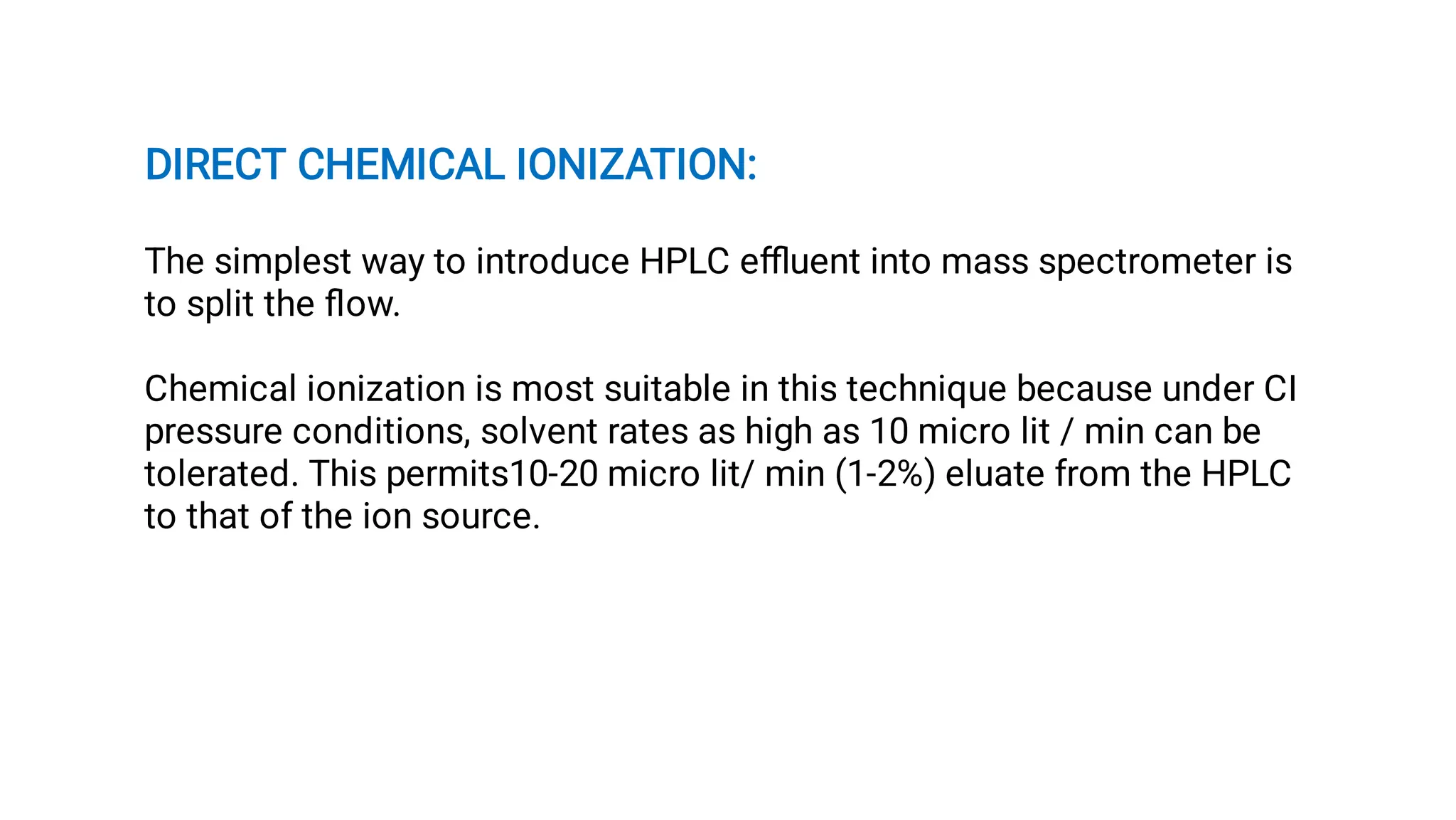
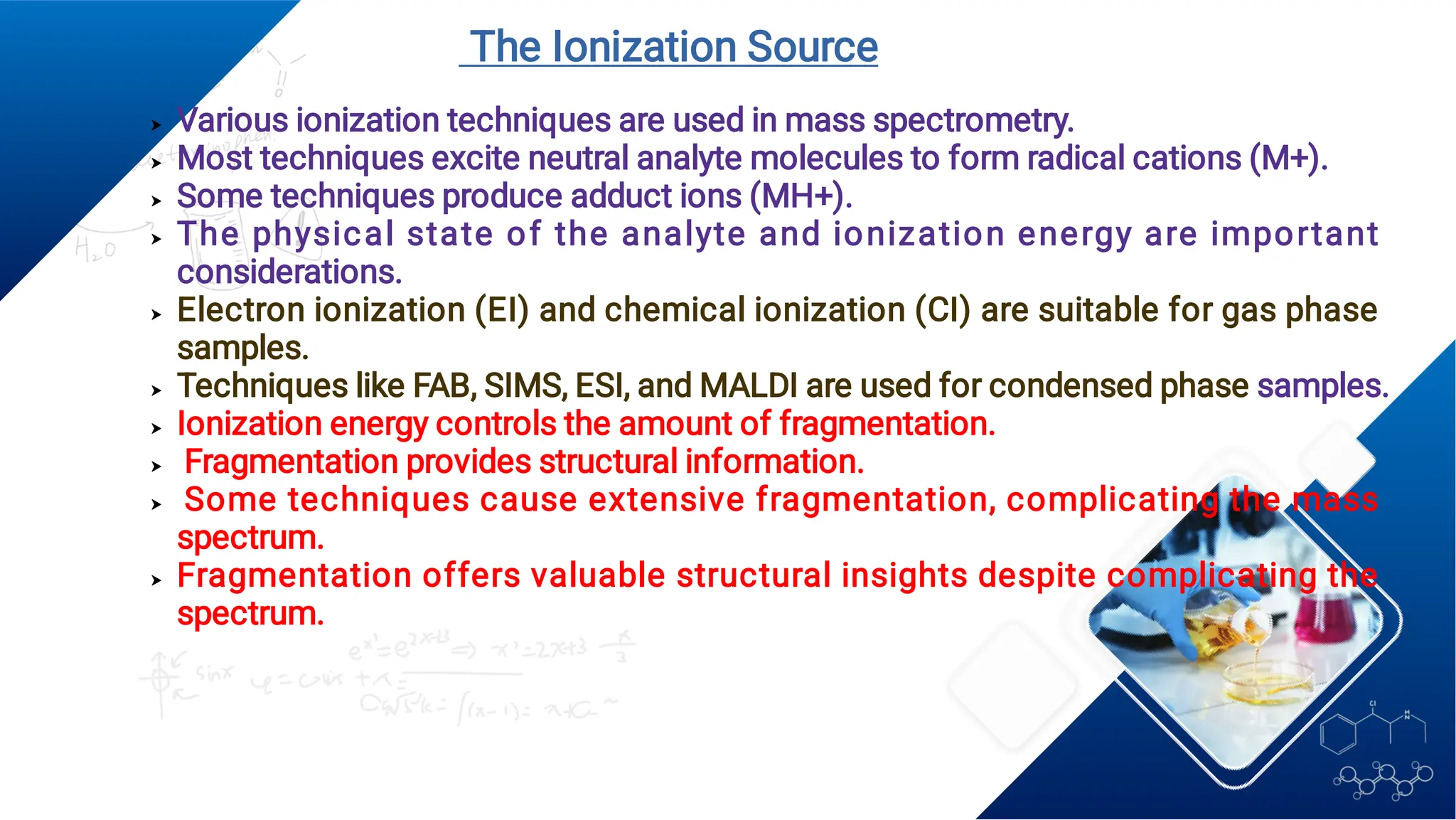
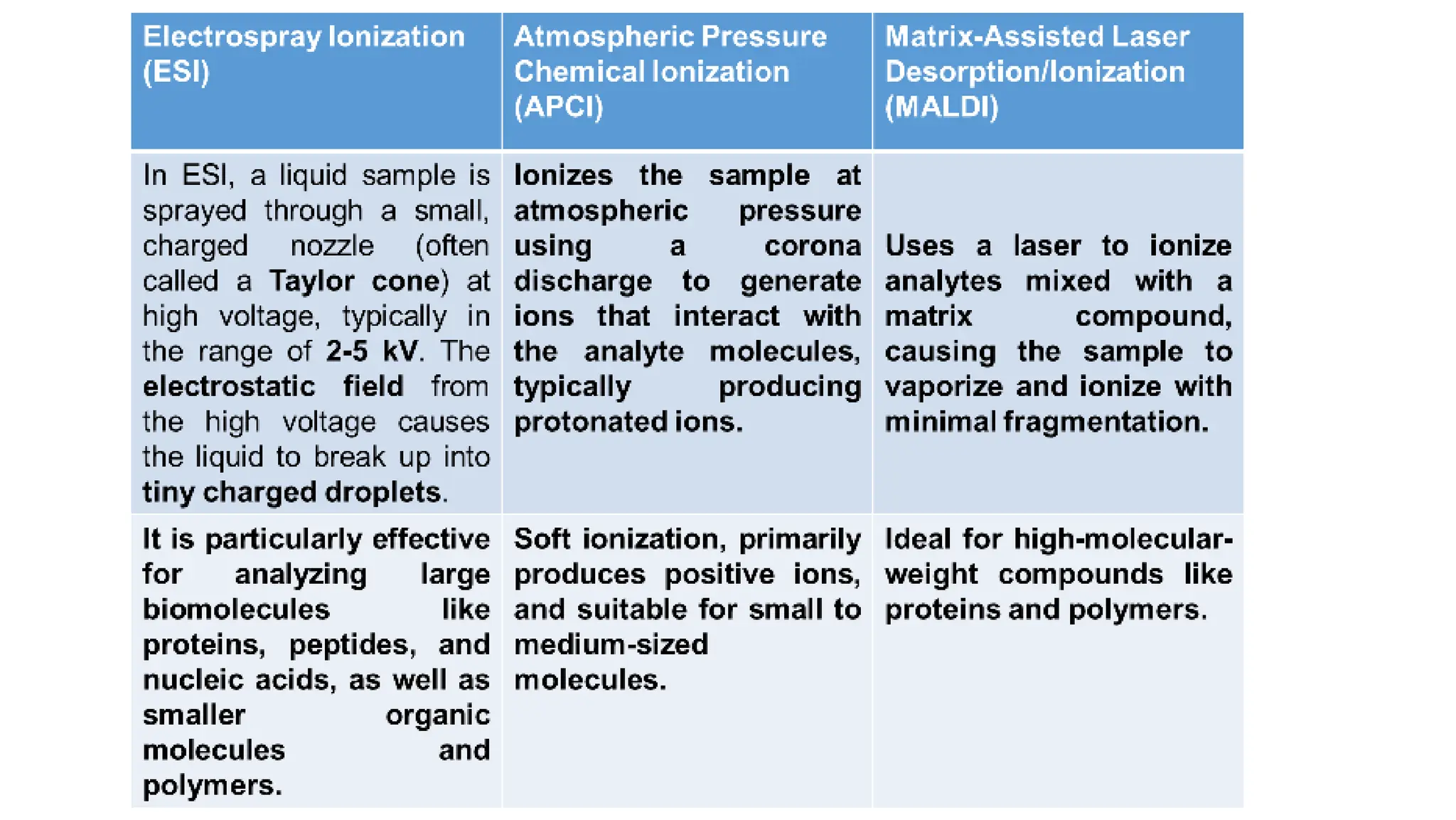
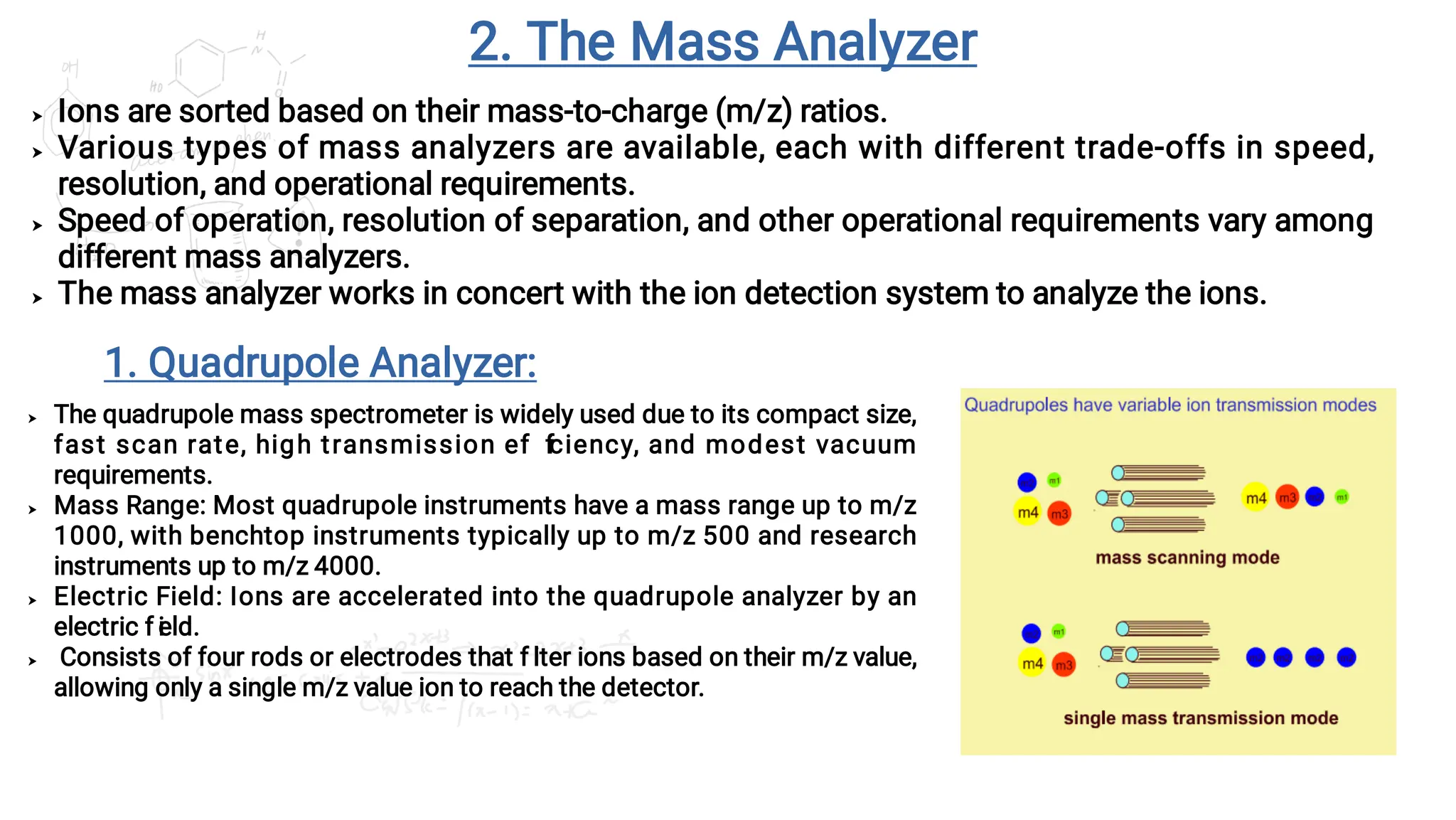
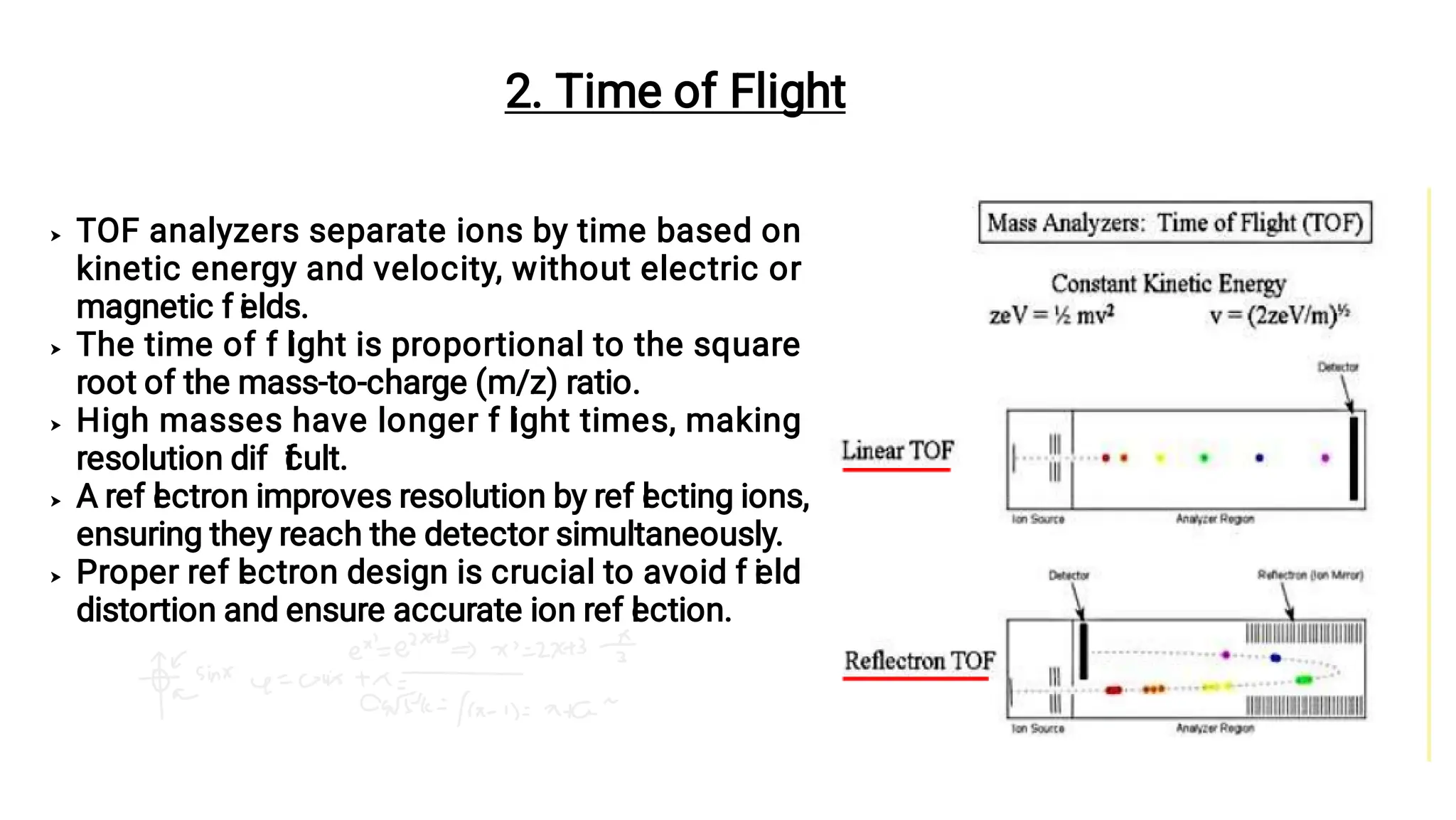
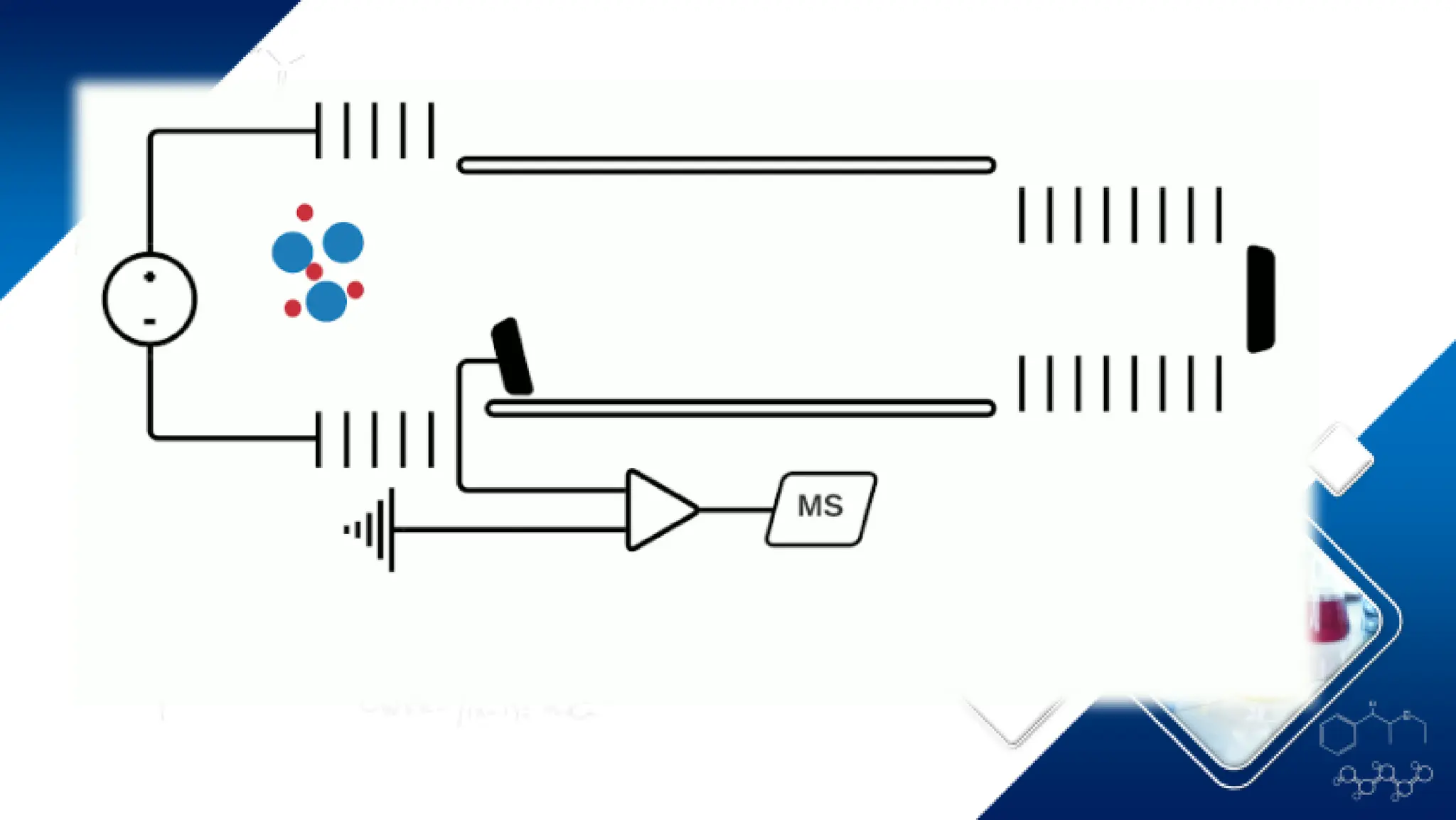
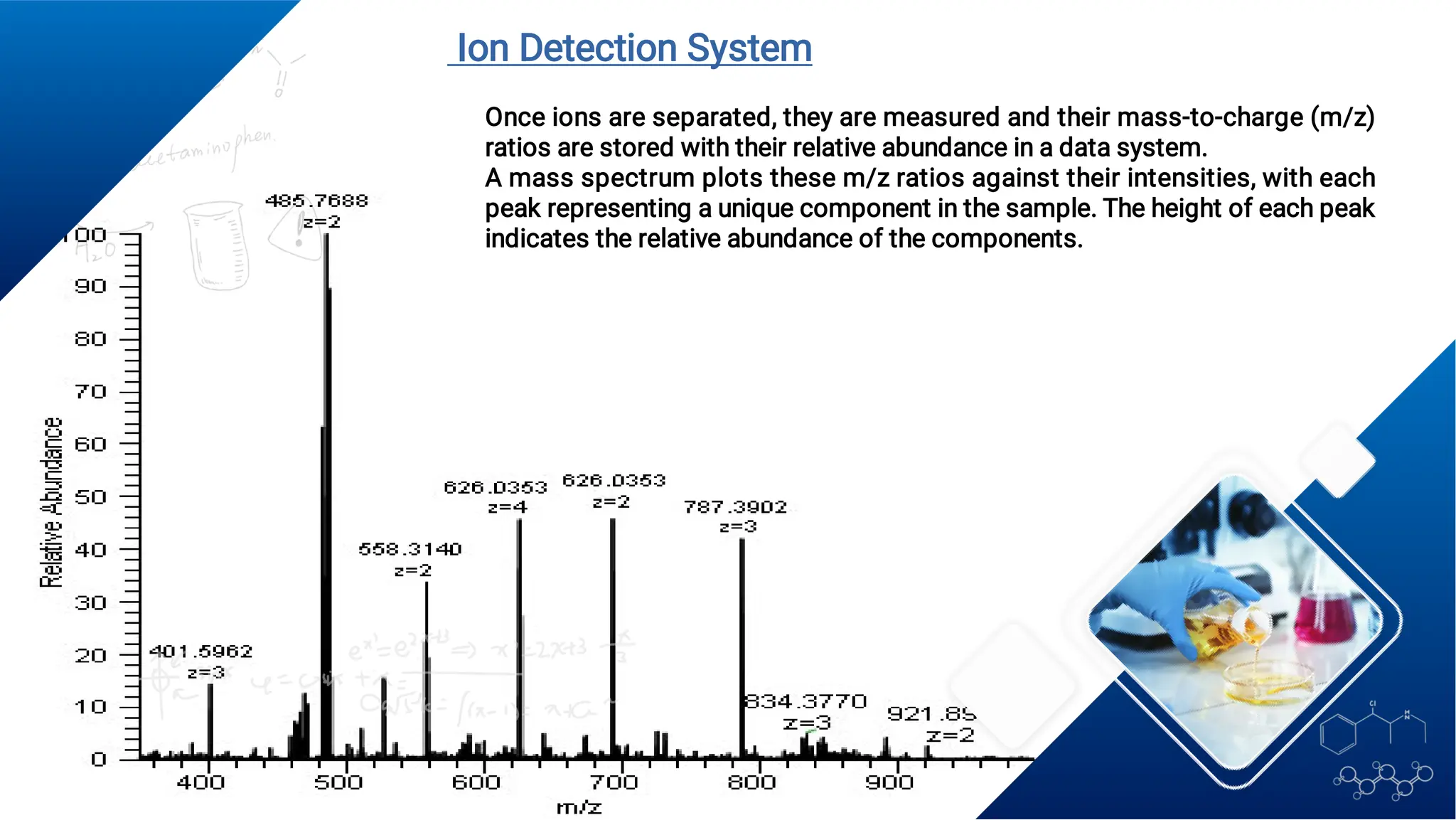
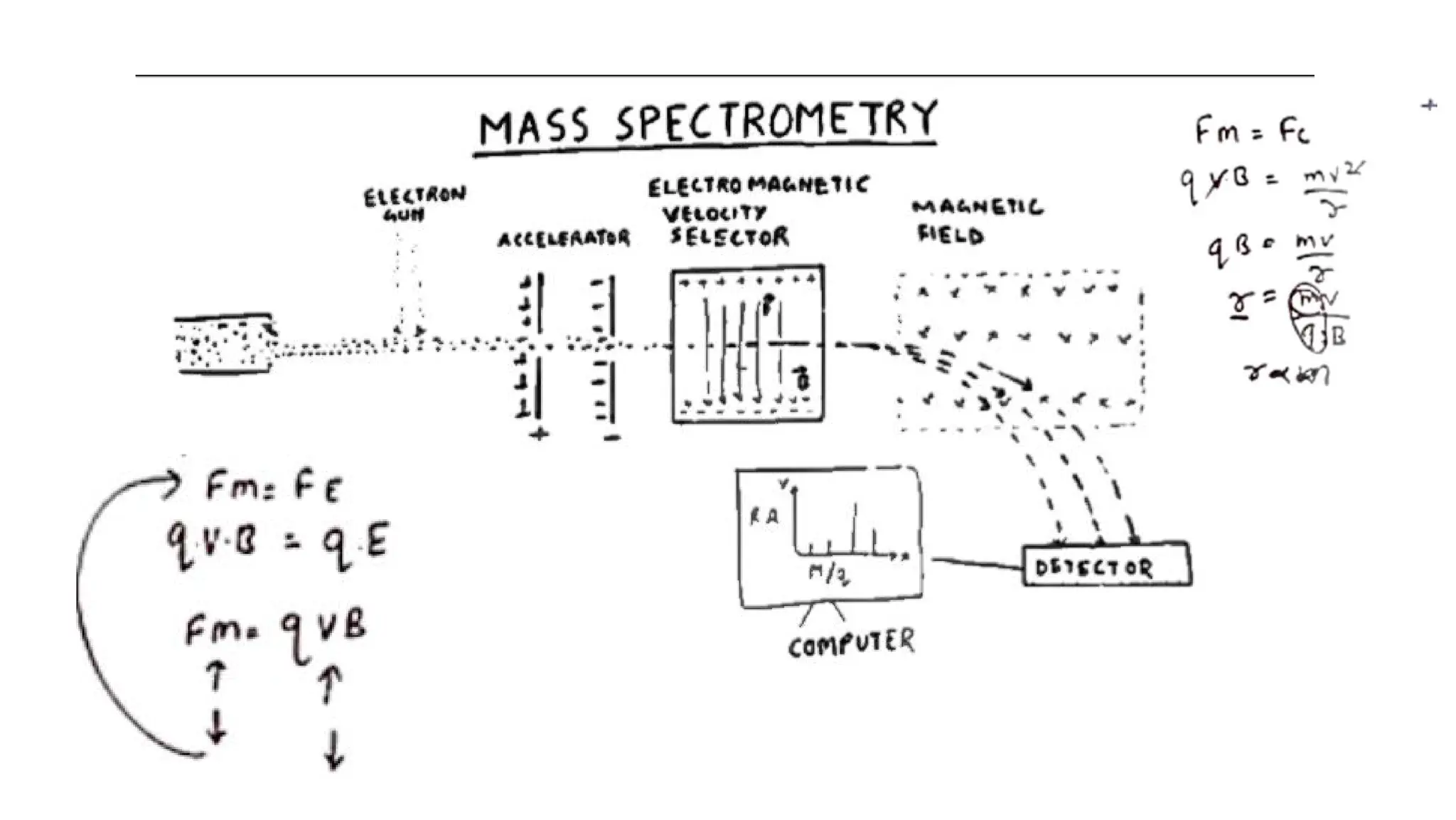
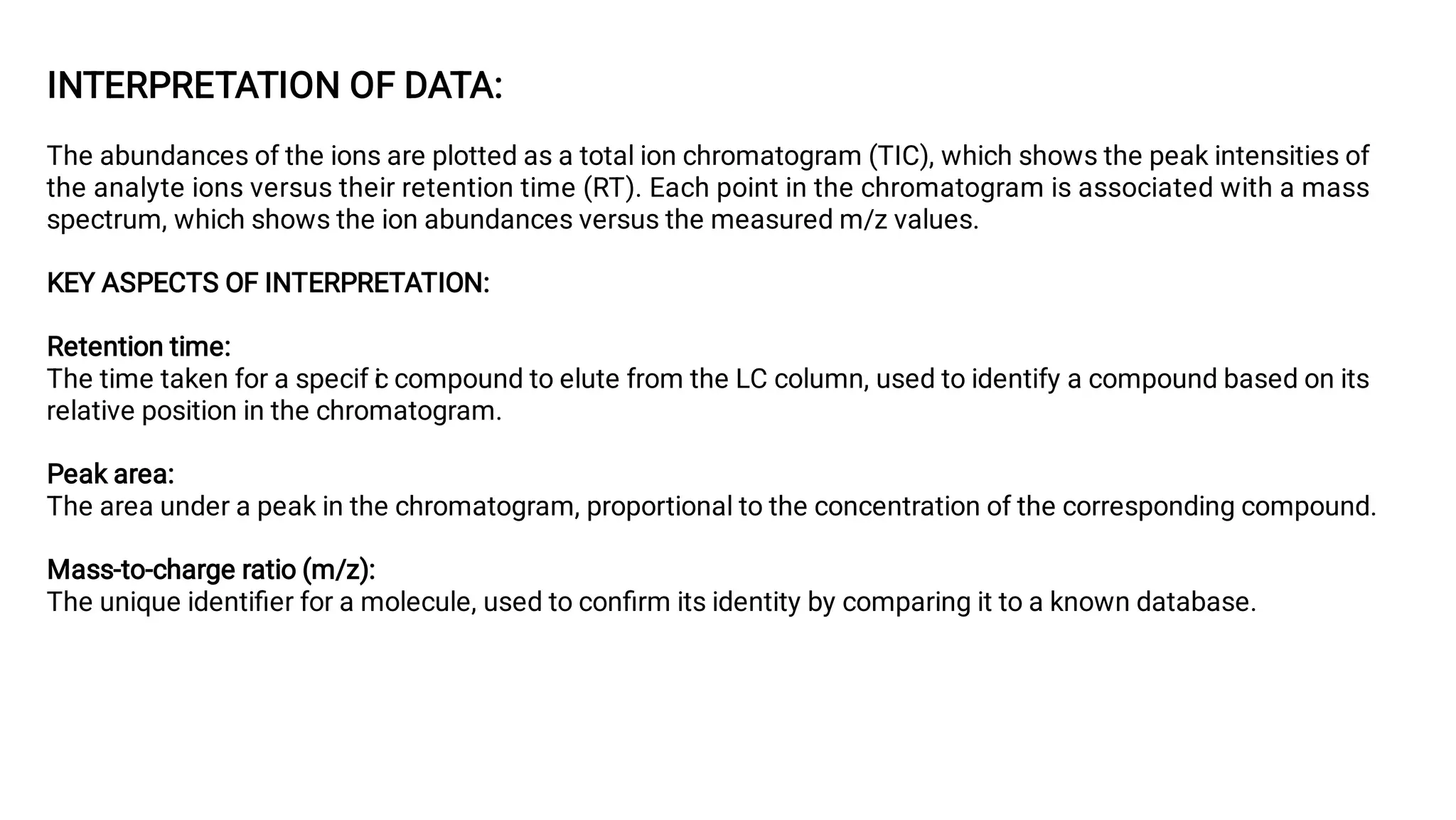
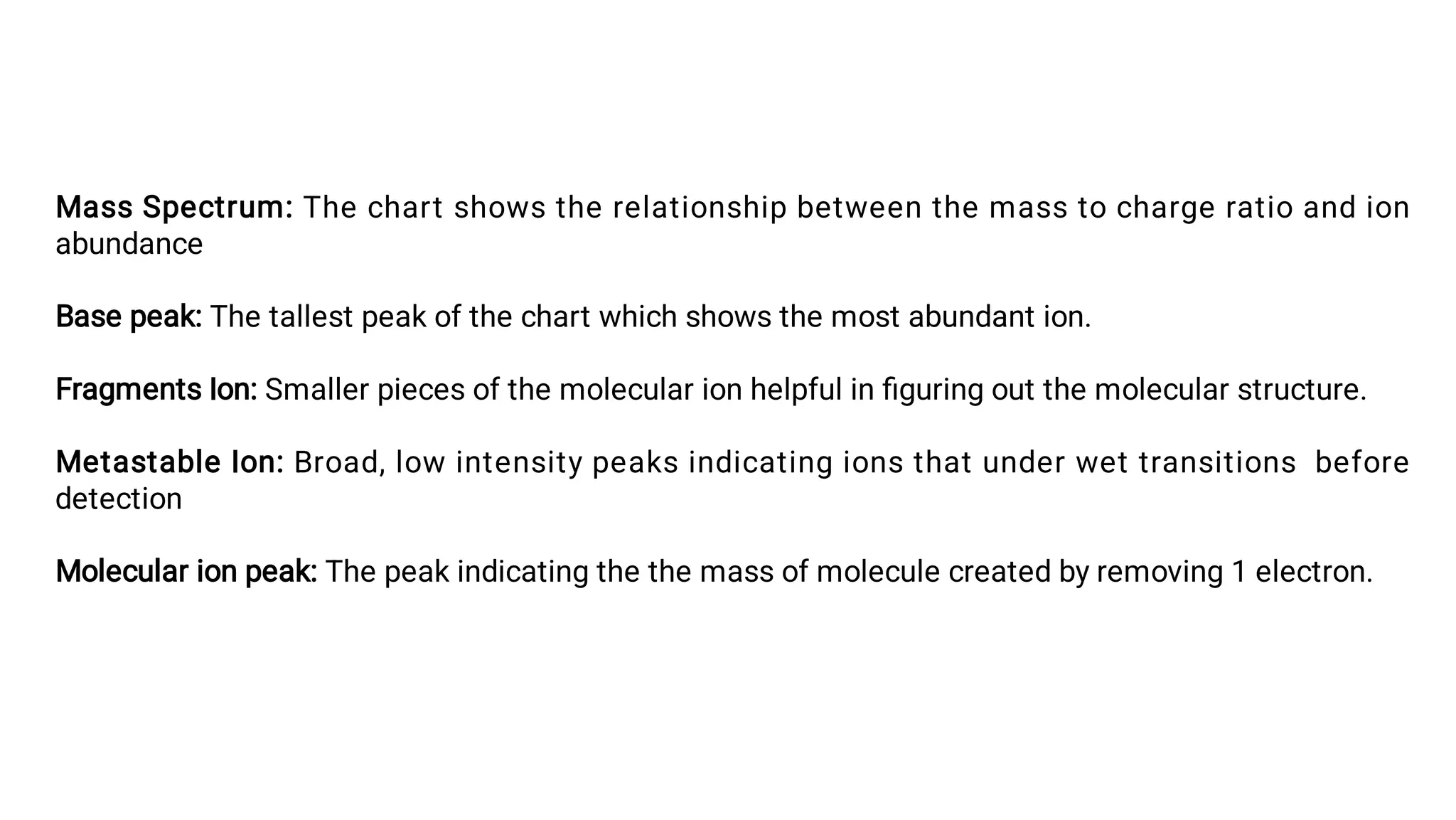
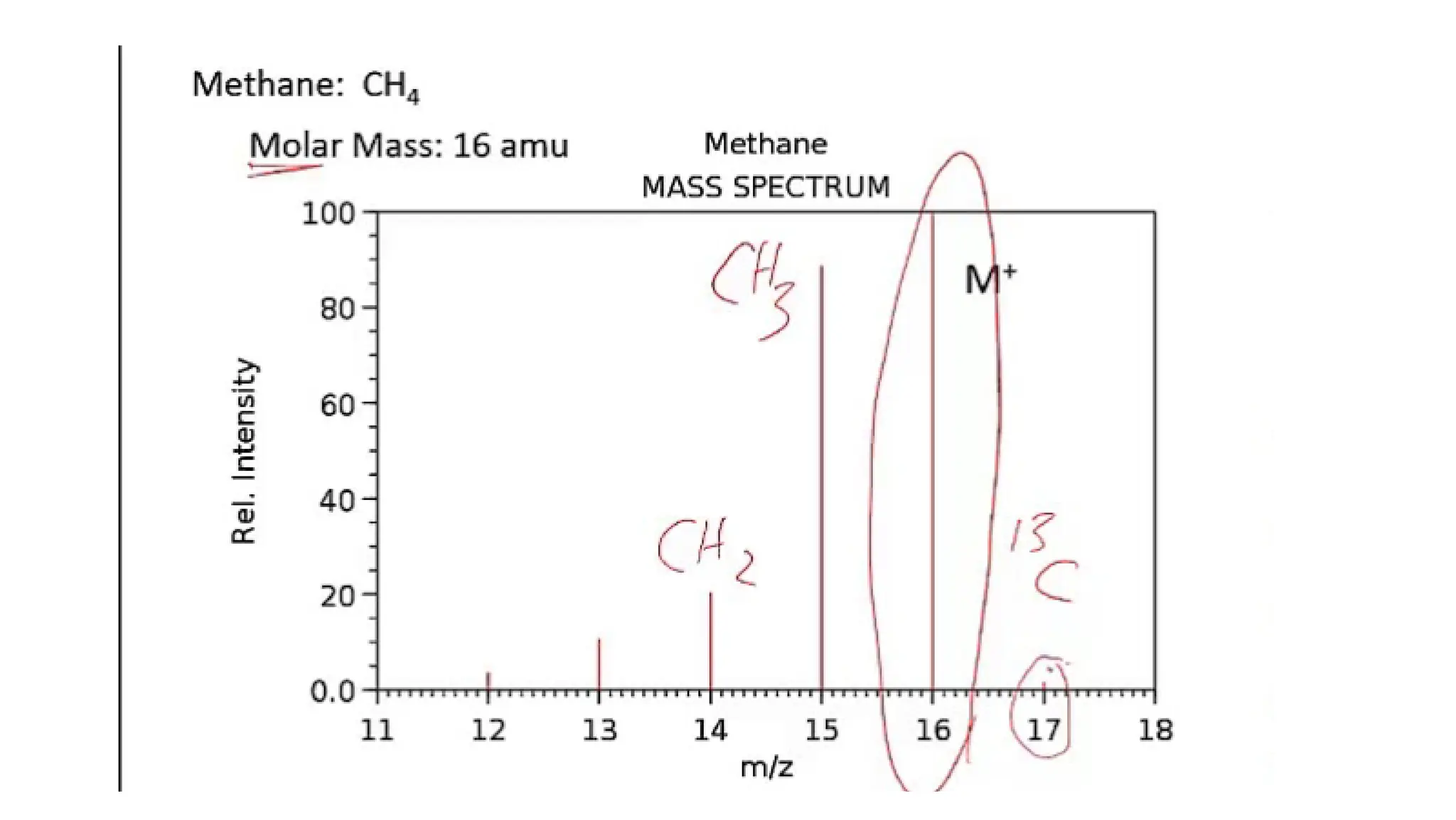
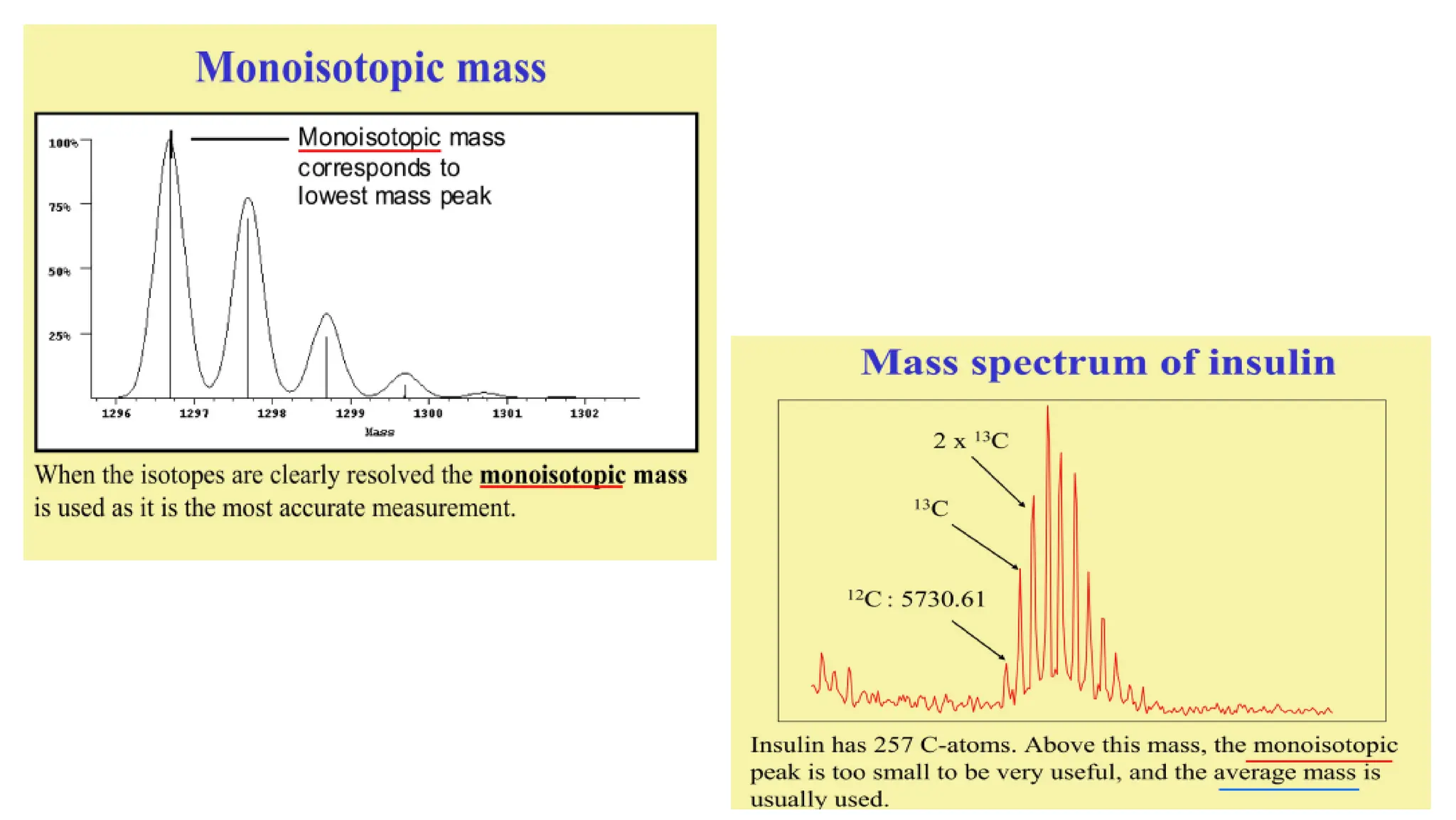
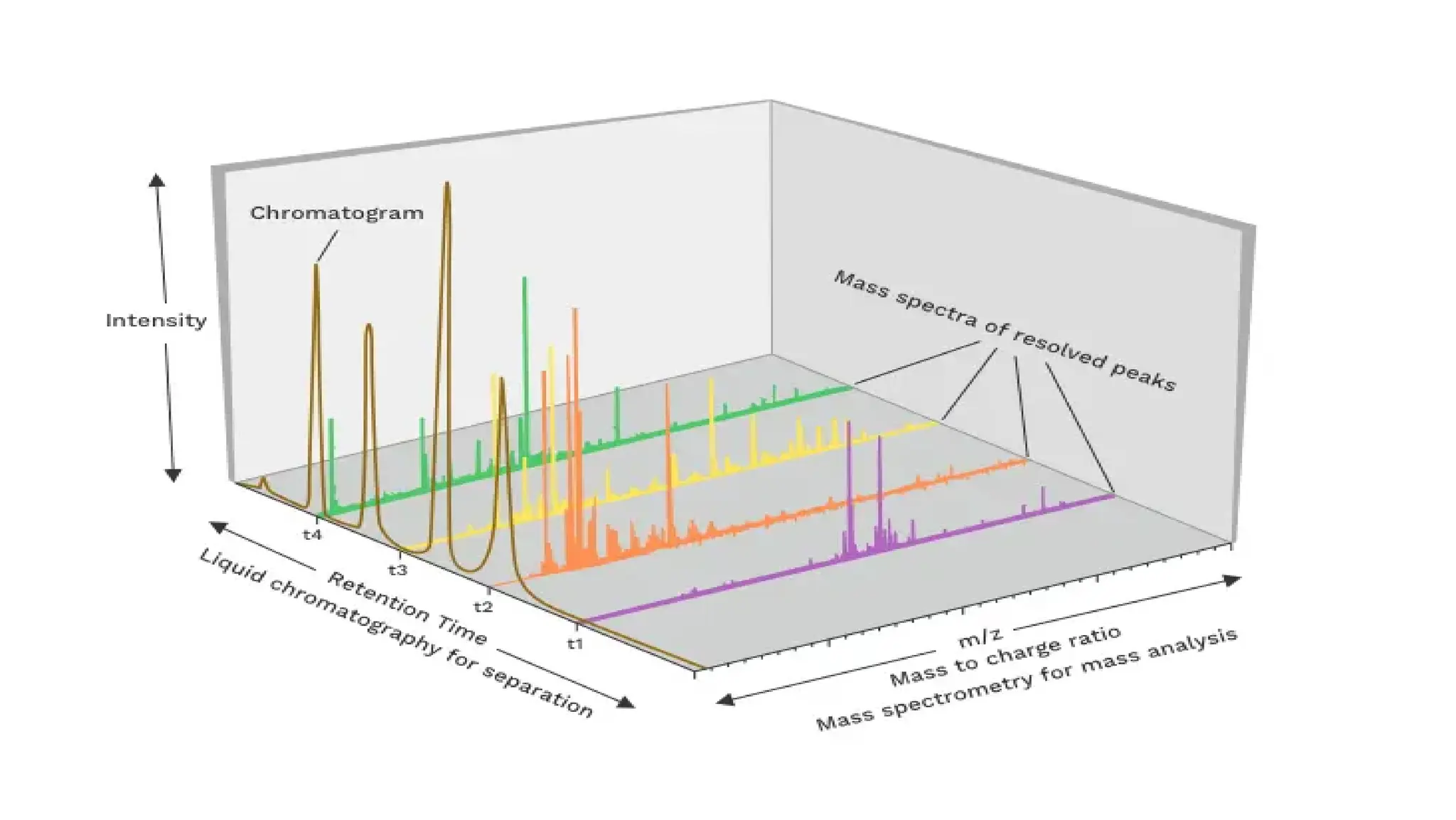
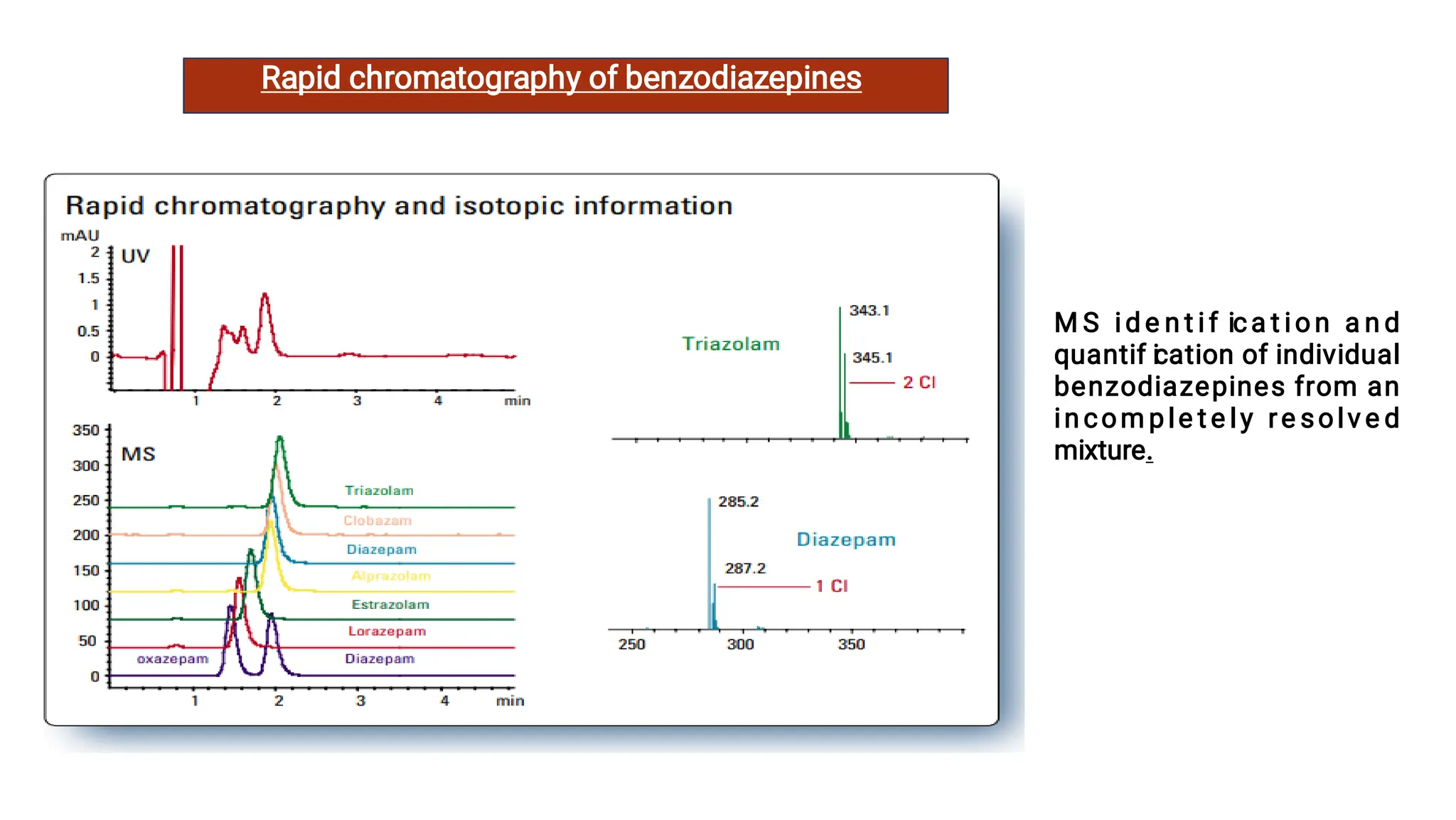
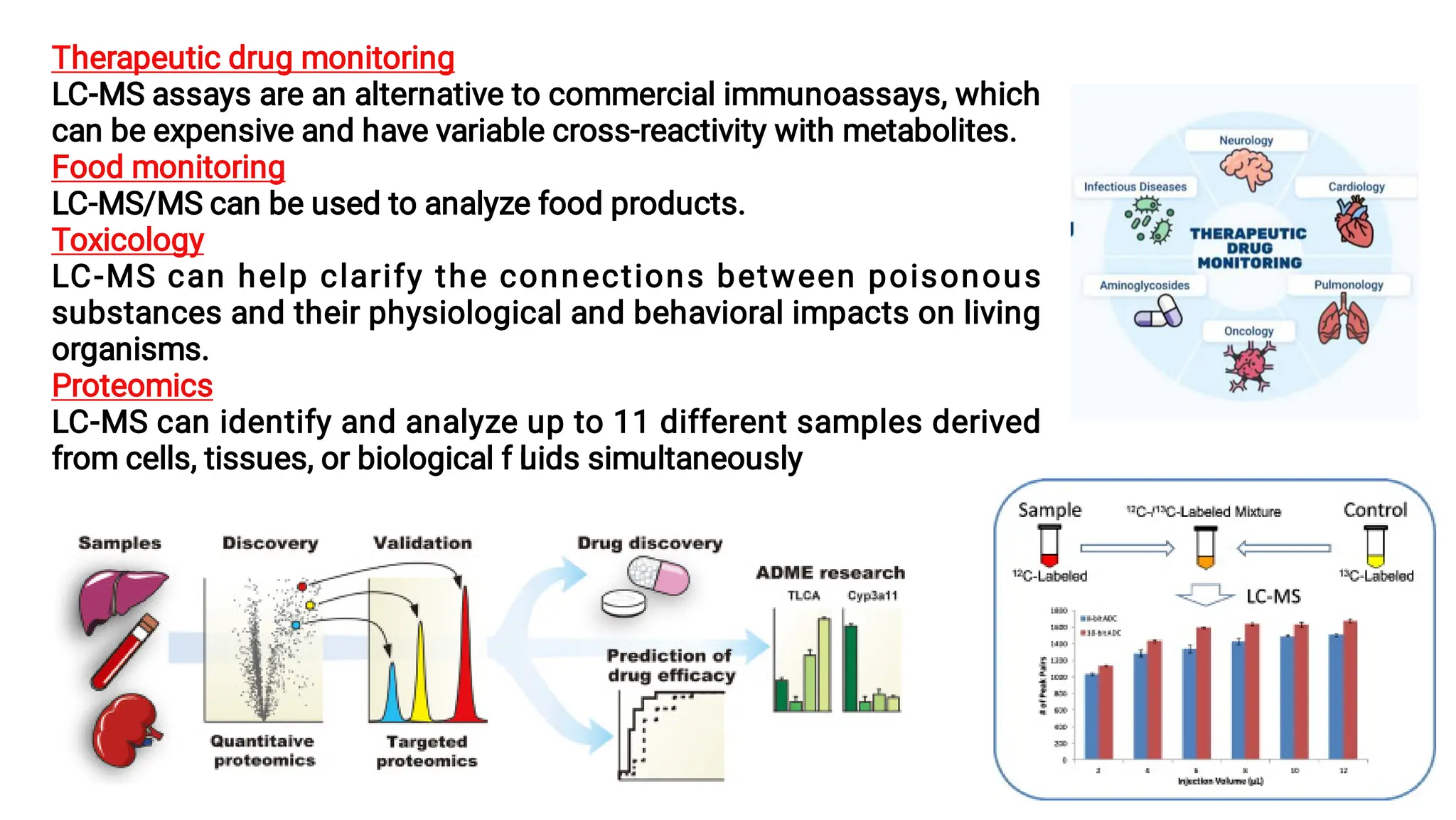
Liquid chromatography/mass spectrometry (LC-MS) is a powerful analytical technique that combines liquid chromatography's separation capabilities with mass spectrometry's detection abilities for qualitative and quantitative analysis of compounds. It utilizes various ionization techniques and mass analyzers to provide sensitive, accurate measurements of compounds in complex mixtures, making it valuable across a wide range of applications including drug detection, environmental analysis, and biomarker discovery. The technique's advantages include high sensitivity, selectivity, and the ability to generate comprehensive structural information of the analyzed substances.