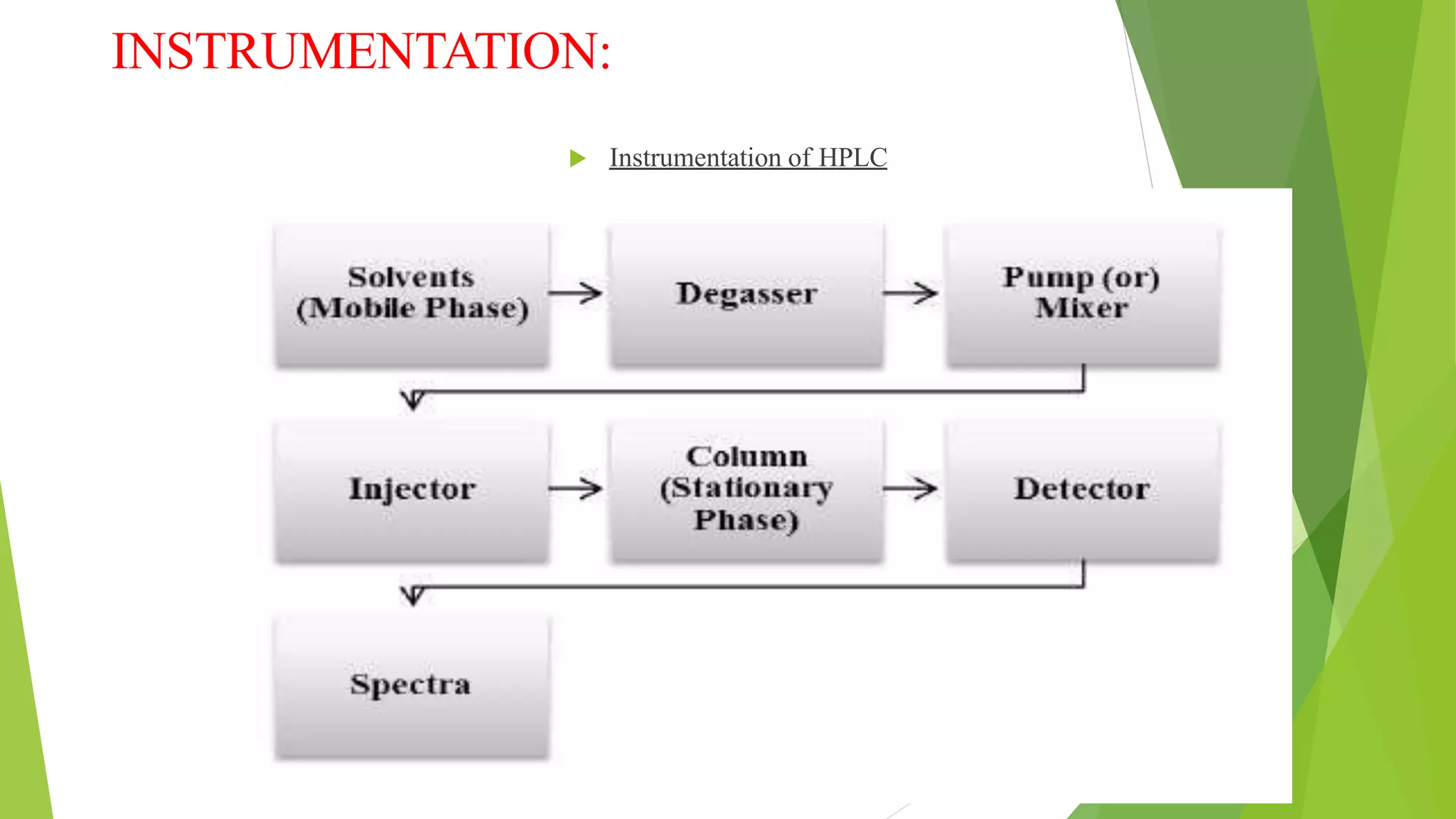
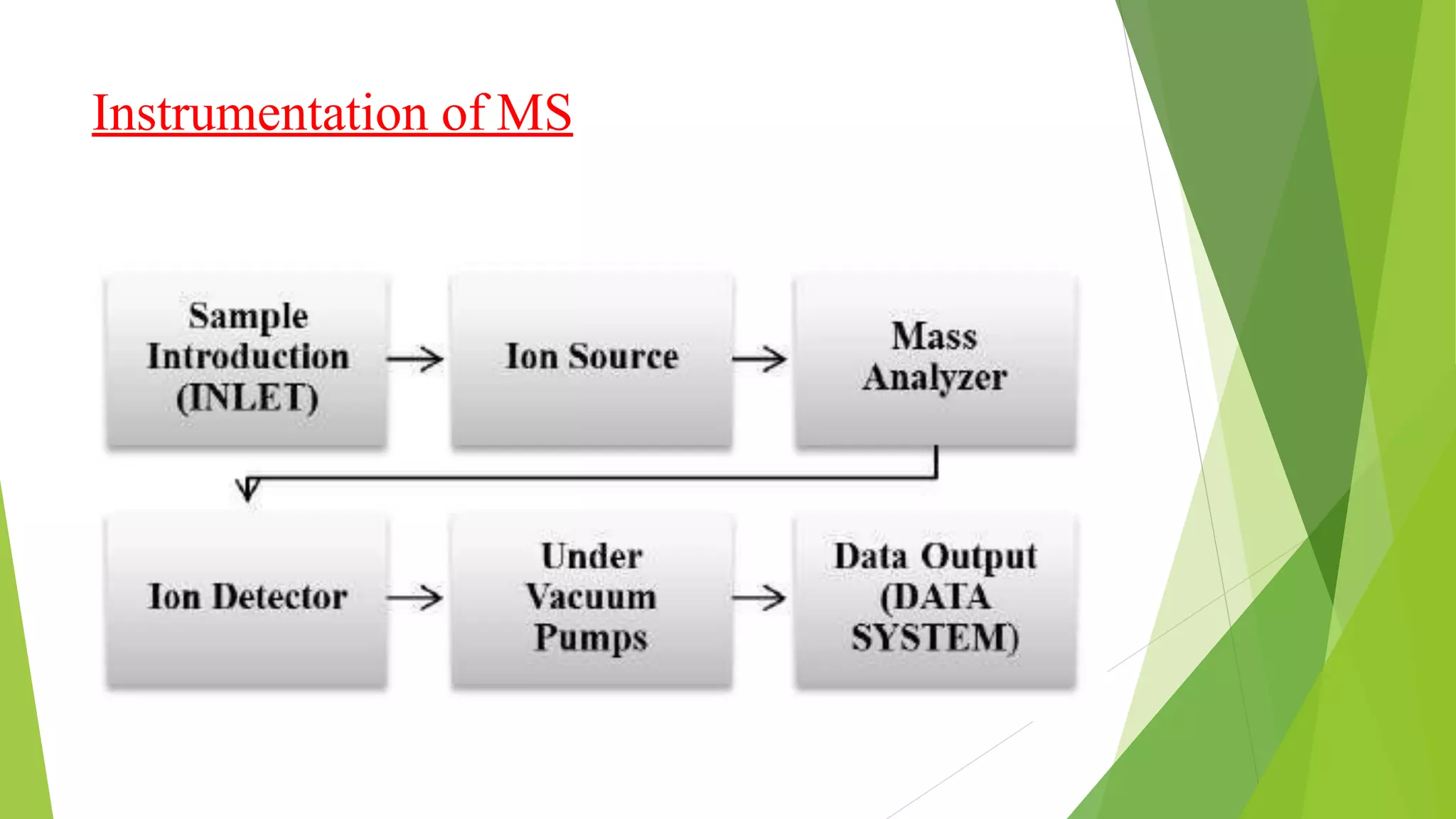
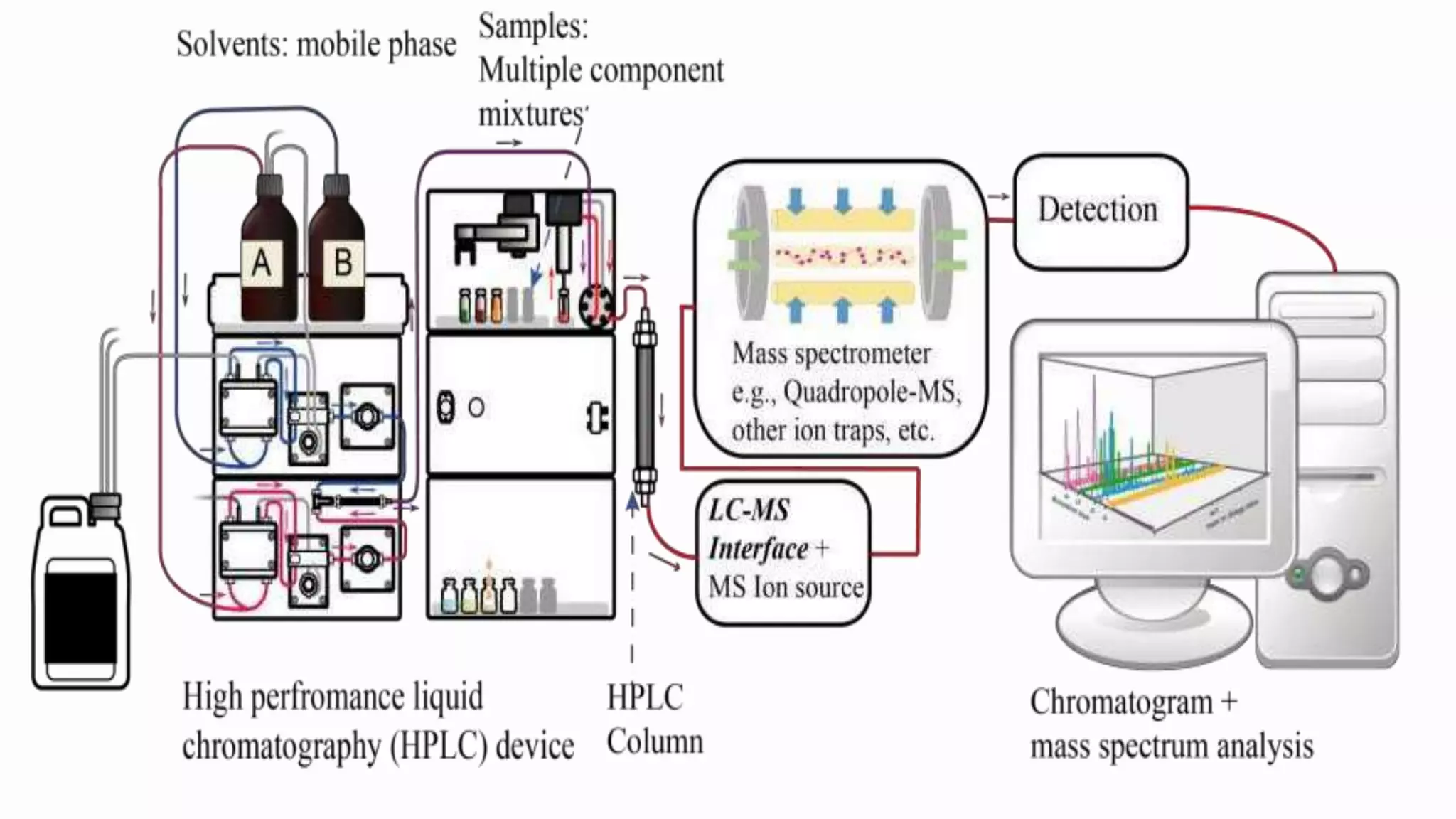
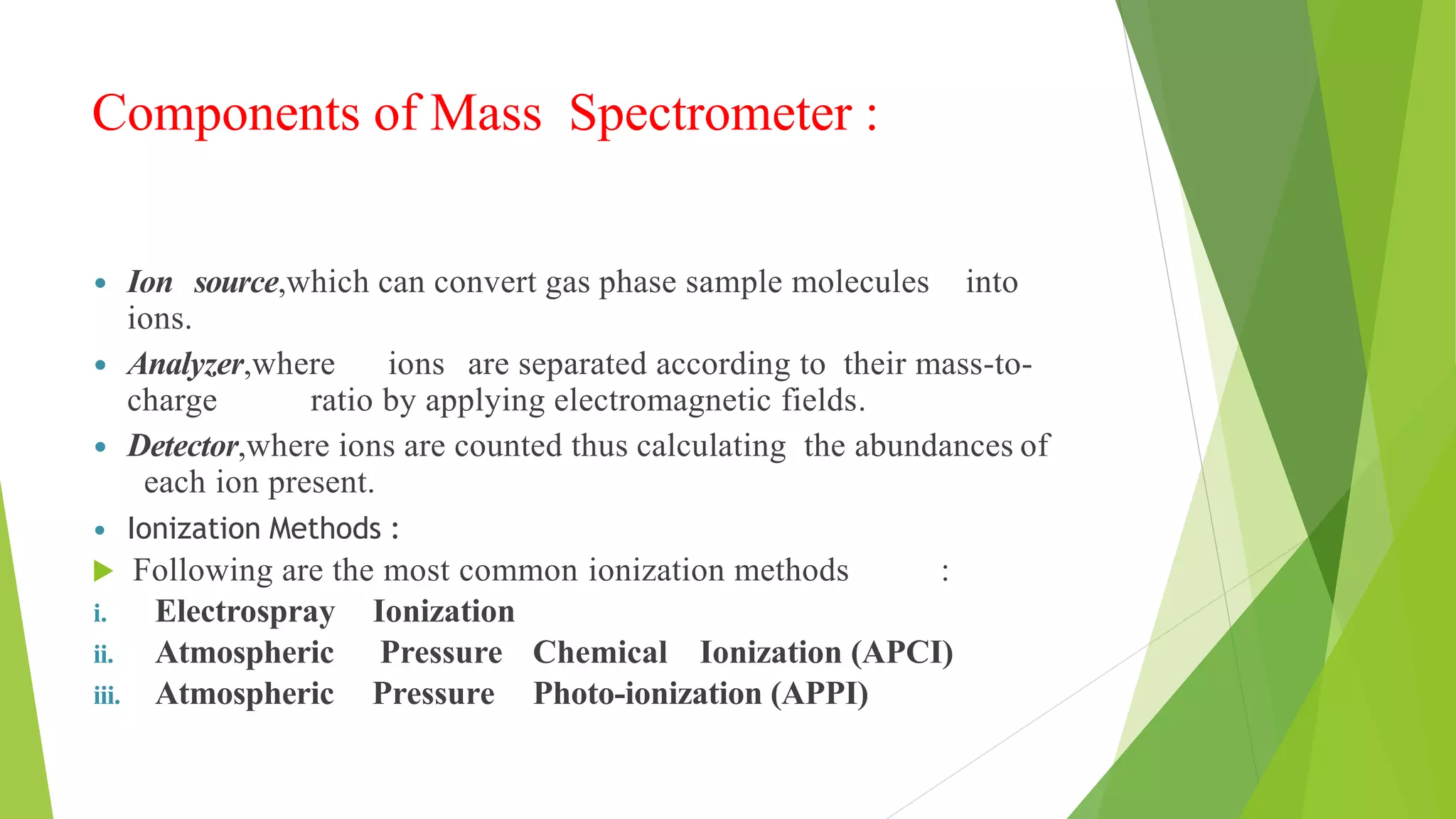
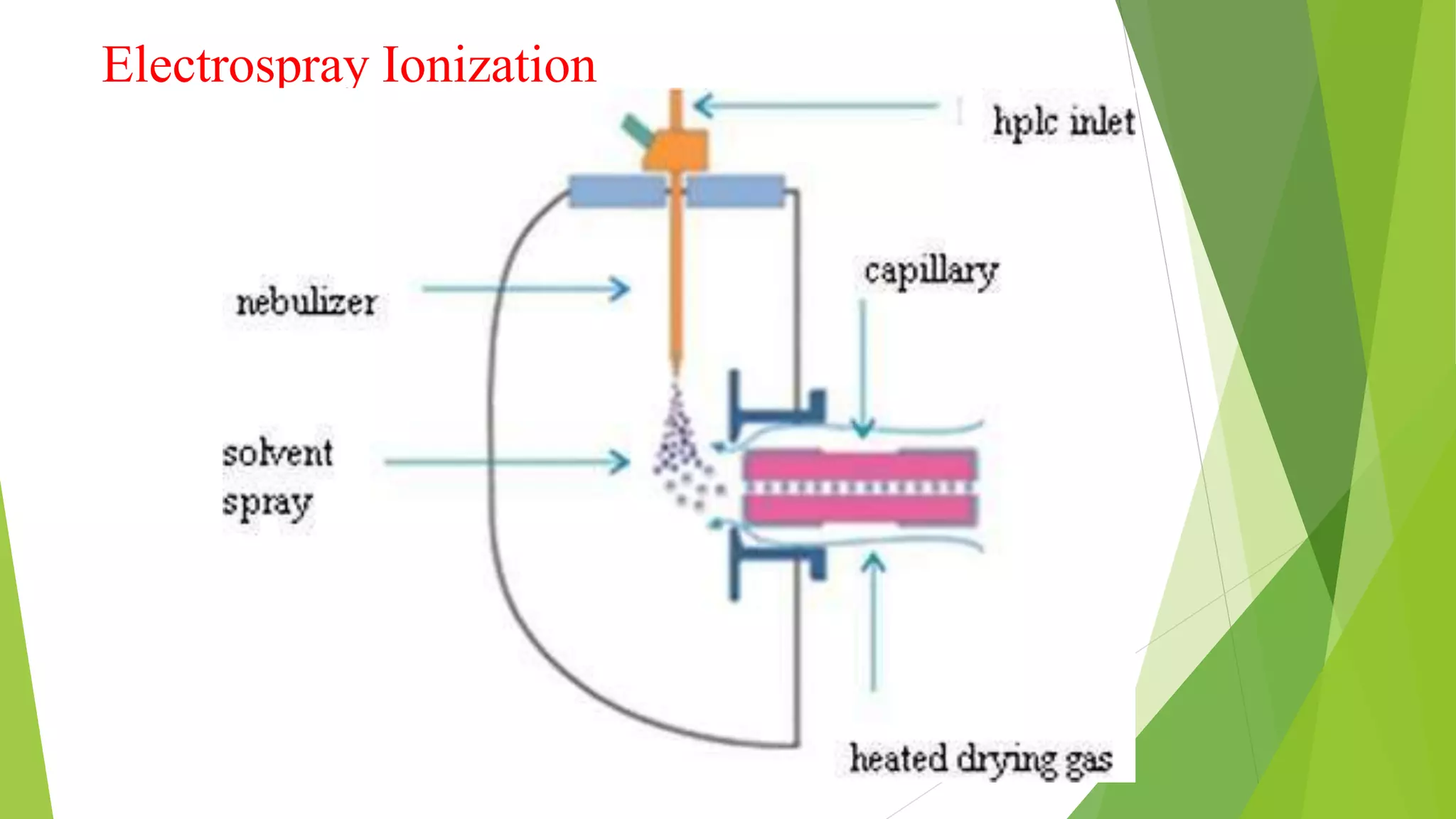
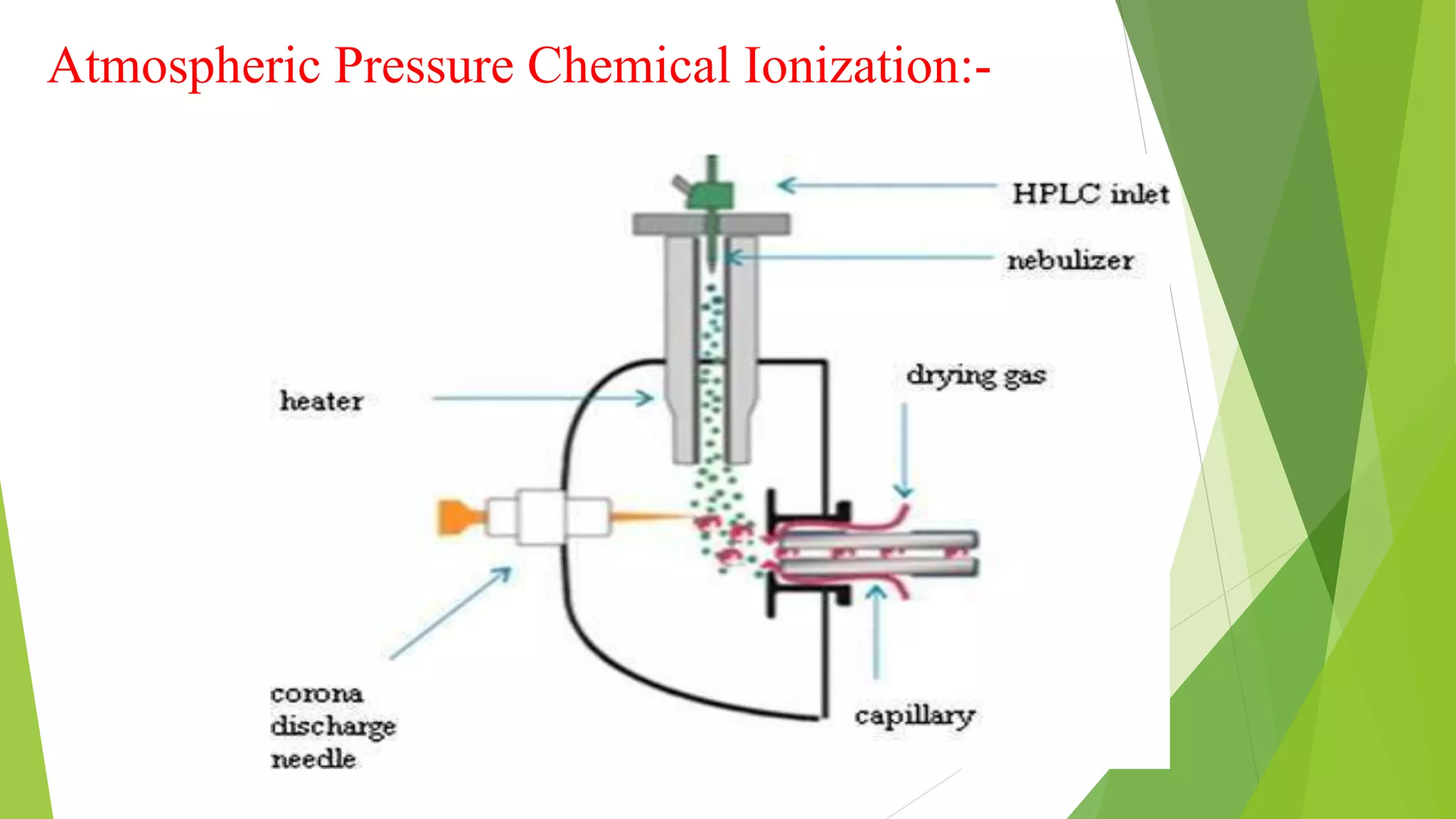
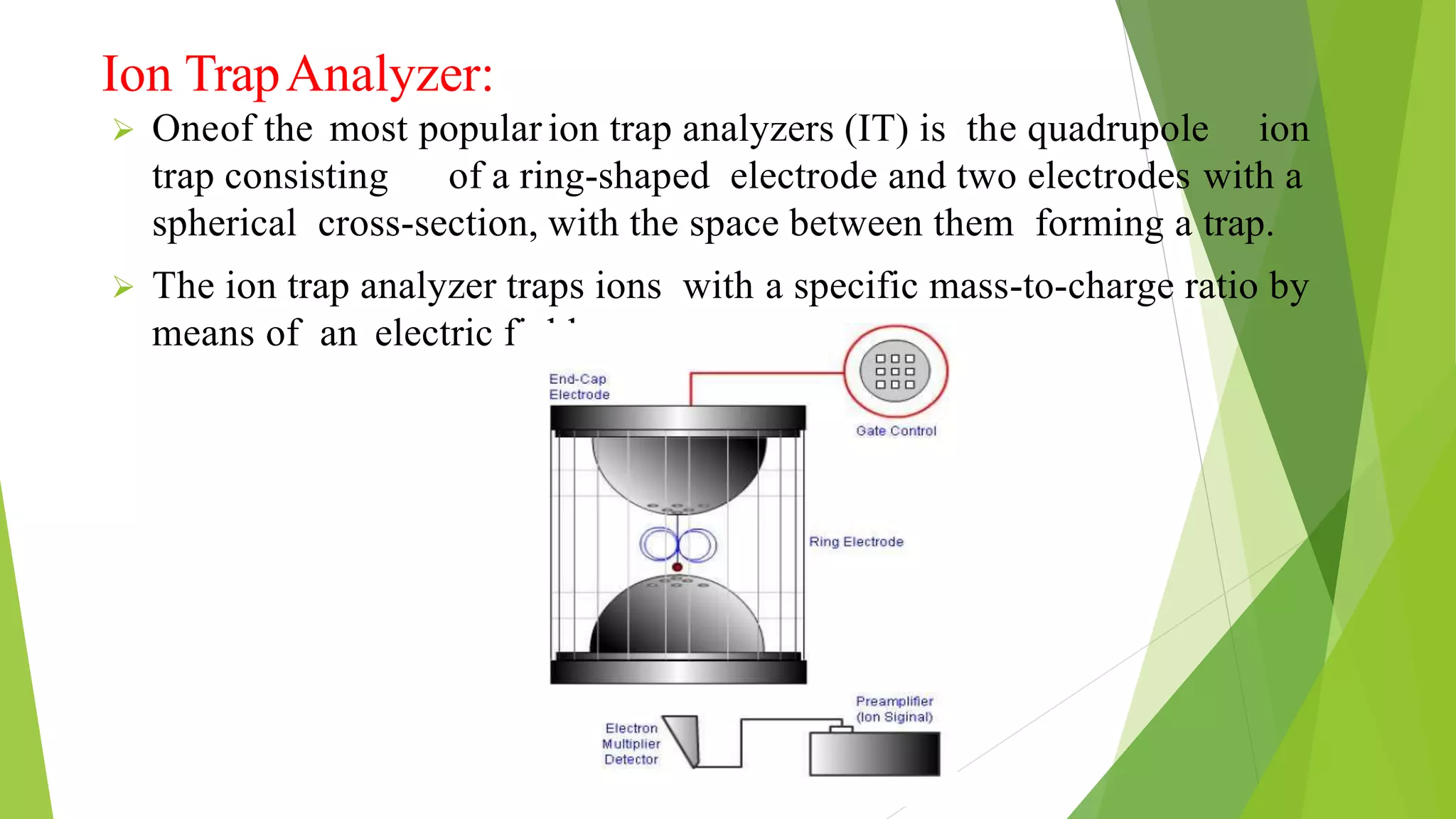
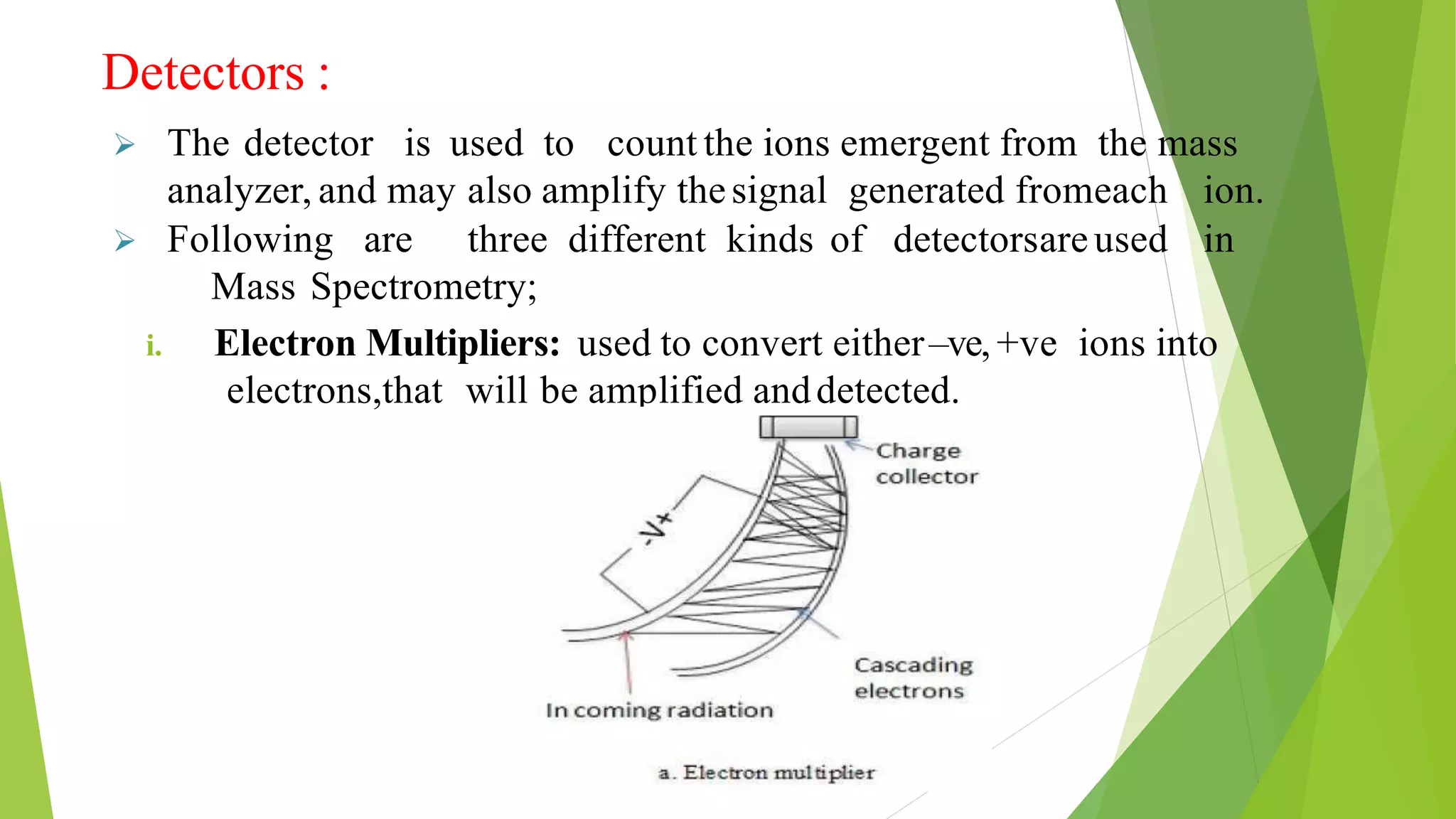
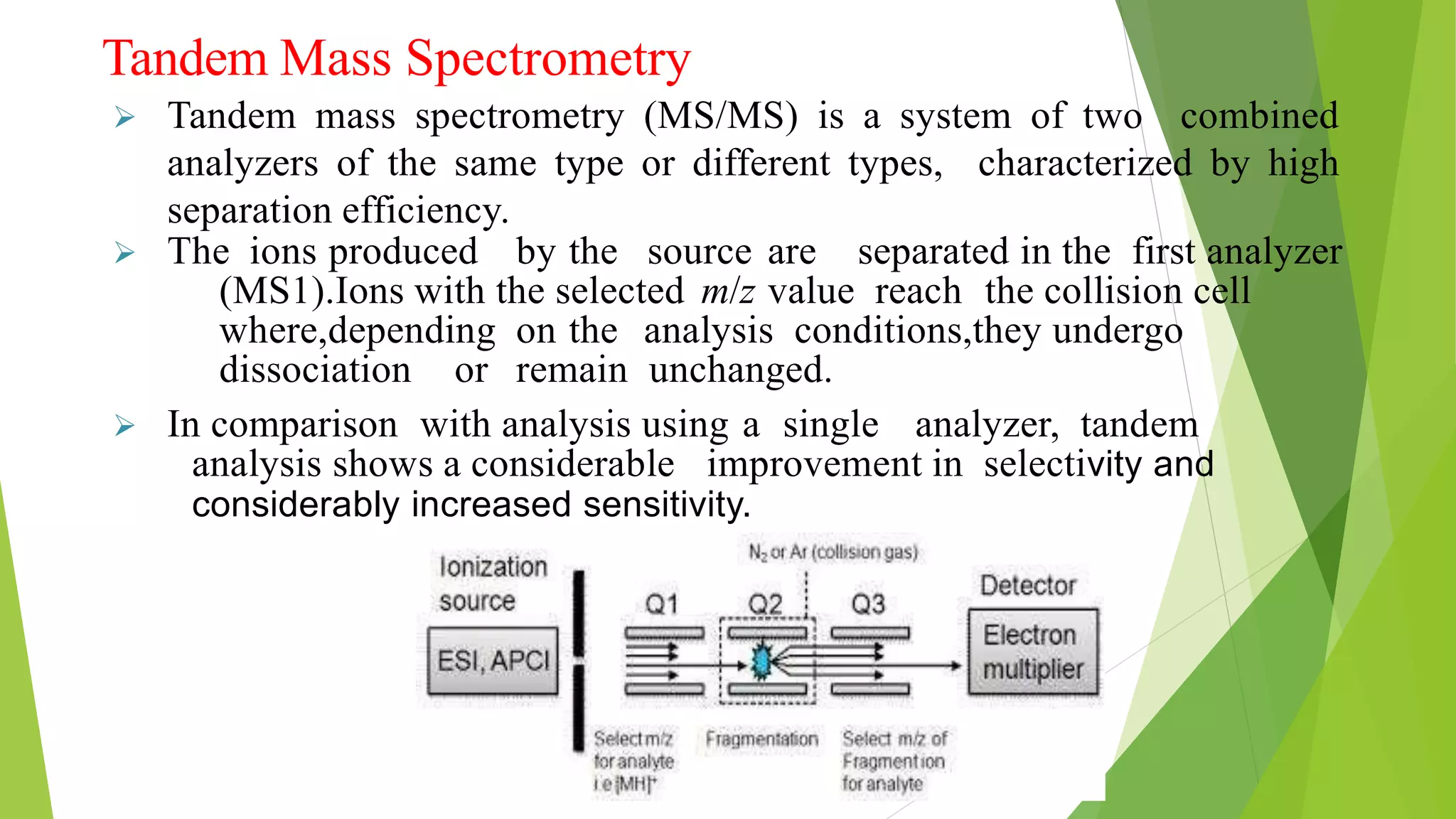
Liquid chromatography-mass spectrometry (LC/MS) combines liquid chromatography with mass spectrometry to separate and identify compounds. It works by separating compounds using liquid chromatography and then using mass spectrometry to identify the compounds by measuring their mass-to-charge ratios. The main components are an HPLC system, ionization source, mass analyzer, and detector. Common applications include identification of unknown compounds, analysis of complex mixtures like metabolites and lipids, and quantification in fields like pharmaceuticals, food, and environmental analysis.