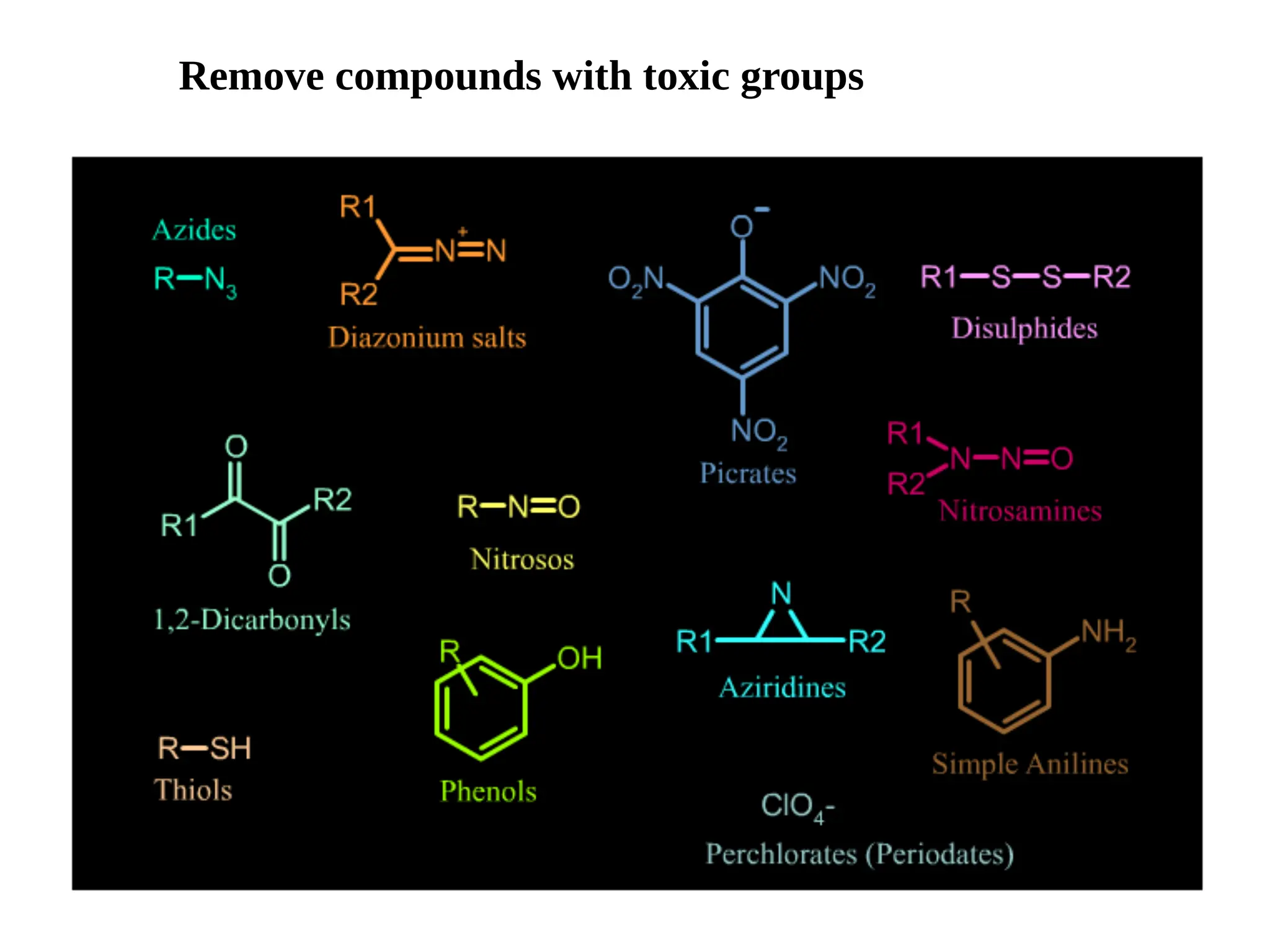
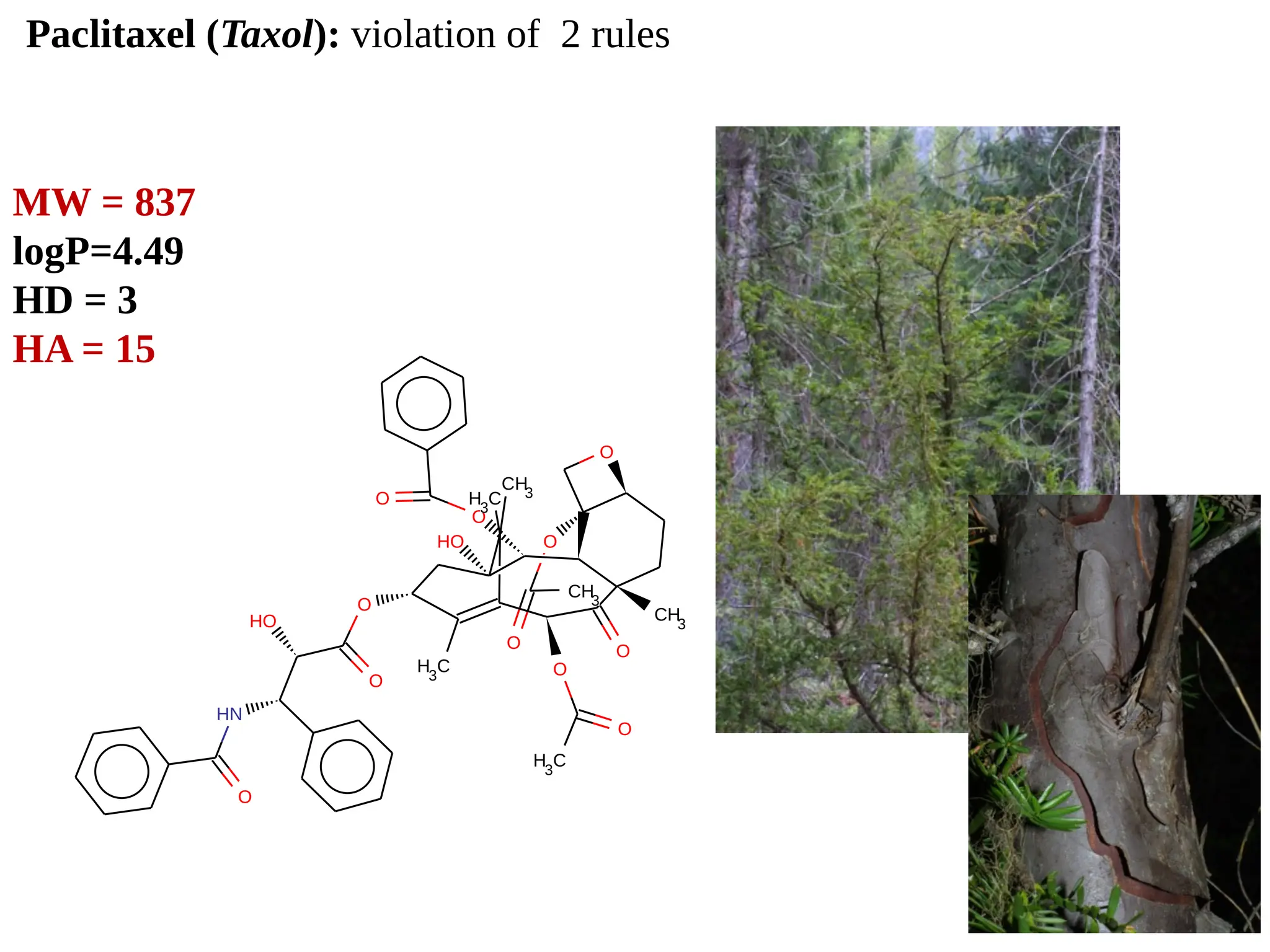
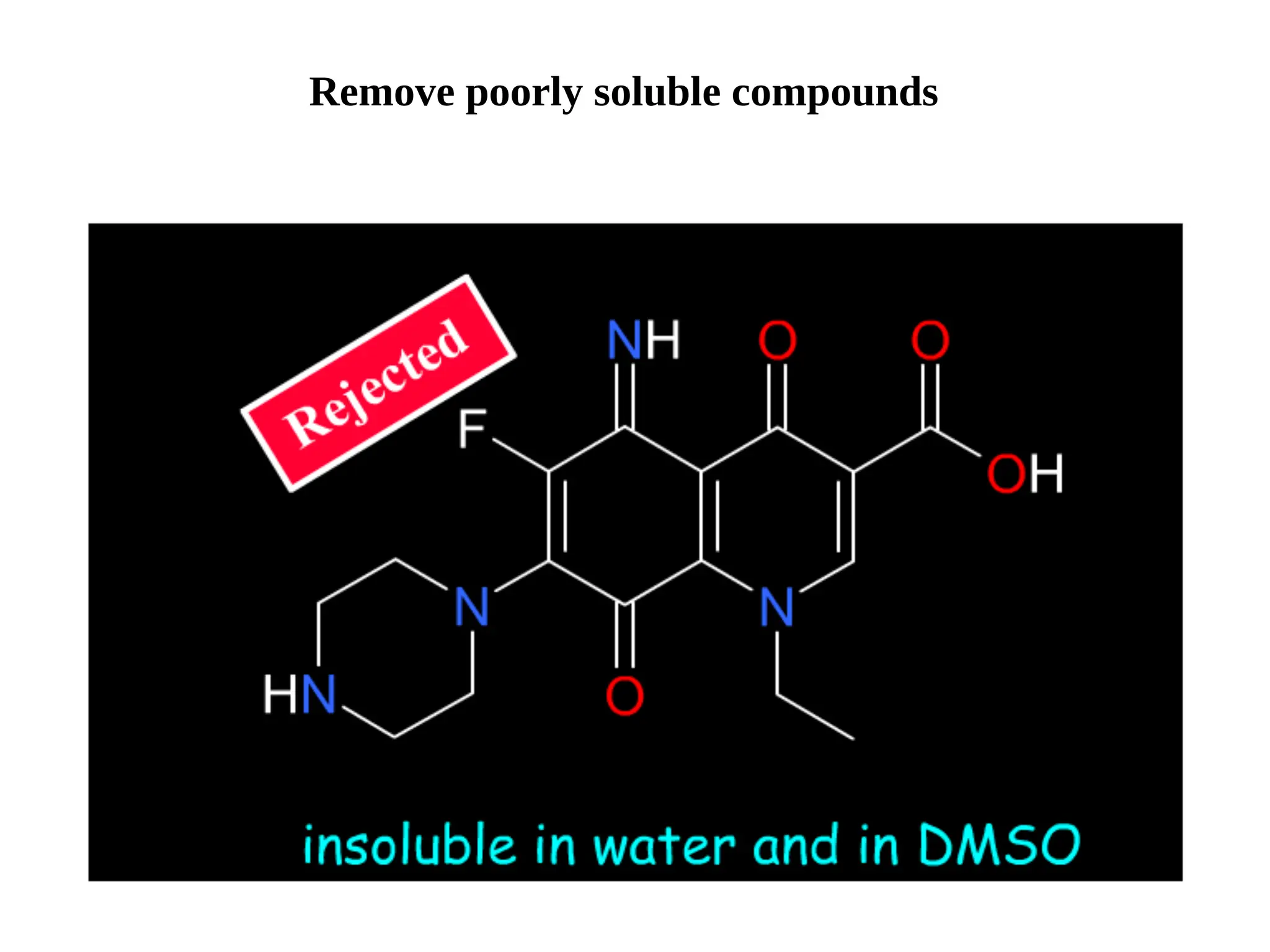
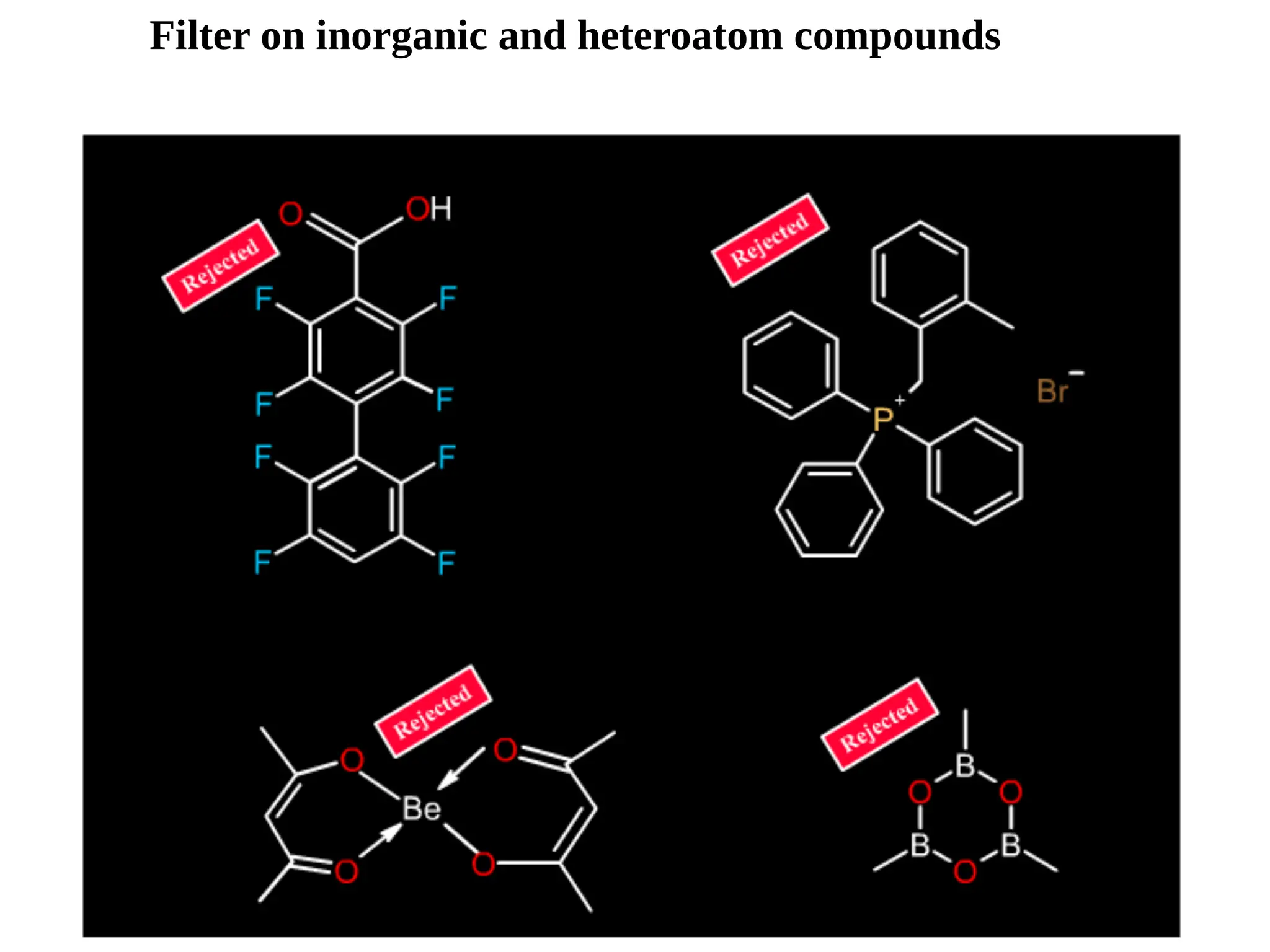
Lipinski's Rule of Five is a guideline to assess the drug-likeness of chemical compounds, formulated in 1997 by Christopher A. Lipinski. It states that an orally active drug generally should not exceed certain criteria: no more than 5 hydrogen bond donors, 10 hydrogen bond acceptors, a molecular mass under 500 daltons, and a log P not greater than 5. The rule aids pharmaceutical and medicinal chemistry students in evaluating drug suitability and designing effective compounds.

![Lipinski's rule of five
Lipinski's rule of five also known as the Pfizer's rule of five
or simply the
Rule of five (RO5) is a rule of thumb to evaluate drug
likeness or determine if a chemical compound with a
certain pharmacological or biological activity has
properties that would make it a likely orally active drug
in humans. The rule was formulated by Christopher A.
Lipinski in 1997, based on the observation that most
medication drugs are relatively small and lipophilic
molecules [Lipinski et al. 1997, 2001 2004].](https://image.slidesharecdn.com/lipinskisrules-240305092356-4c98c6d8/75/Lipinskis-rules-of-five-for-bioavailability-2-2048.jpg)