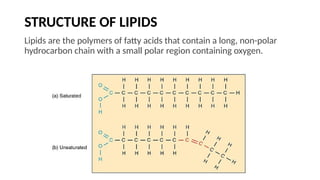
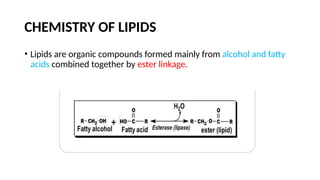
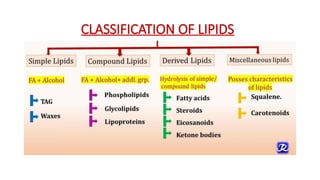
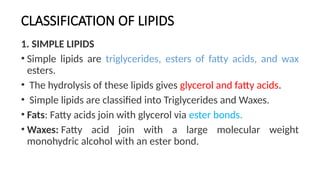
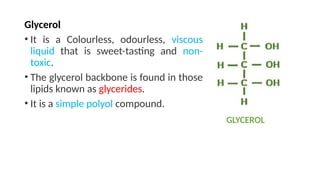
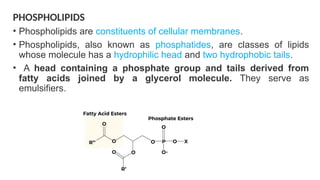
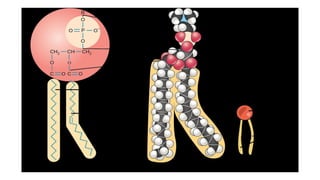
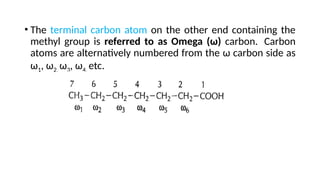
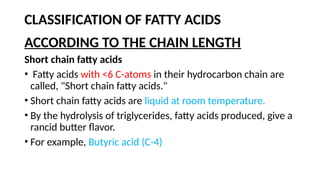
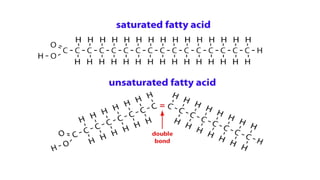
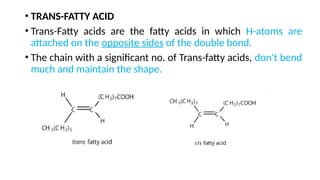
The document discusses lipids, their properties, and classifications such as simple, complex, derived, and miscellaneous lipids, explaining their roles in energy storage, cell structure, and signaling. It details fatty acids along with their classifications based on chain length and degree of unsaturation, highlighting their physical and chemical properties. The document also covers important functions of lipids in biological processes and their significance in health.