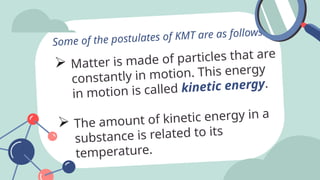
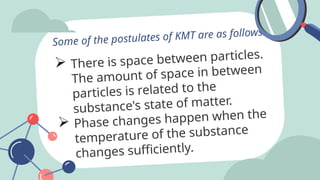
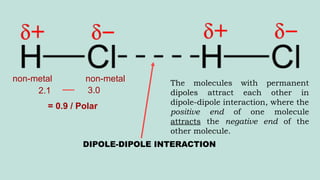
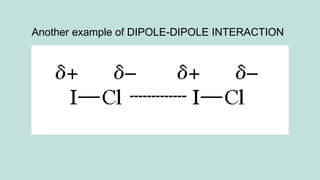
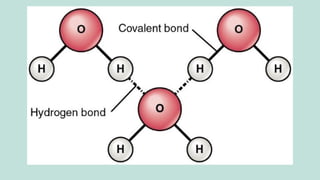
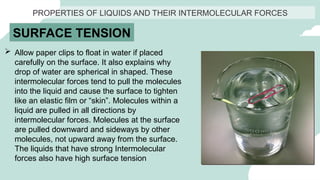
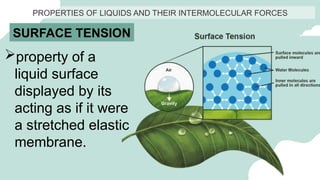
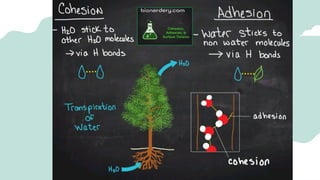
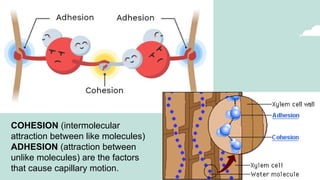
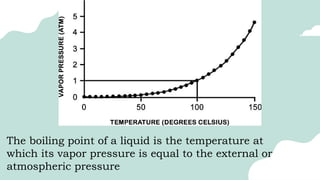
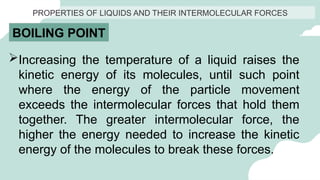
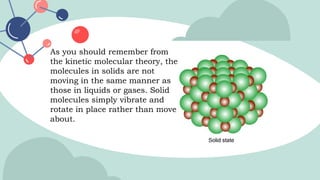
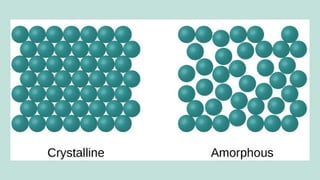
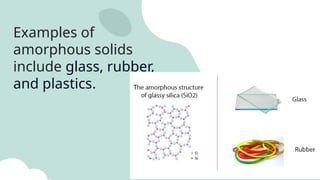
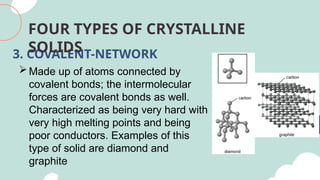
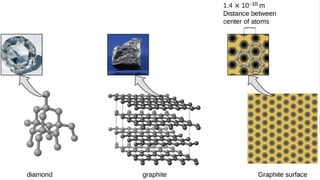
The document outlines the kinetic molecular model and its application in understanding the properties of solids and liquids. It describes the kinetic molecular theory, the types of intermolecular forces (including dipole-dipole, ion-dipole, London dispersion, and hydrogen bonding), and their influence on the physical properties of substances like viscosity, surface tension, and boiling points. Additionally, it discusses the differences between crystalline and amorphous solids and their respective structures and properties.