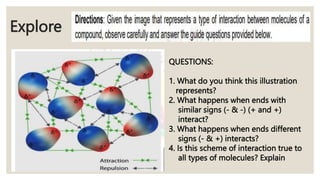
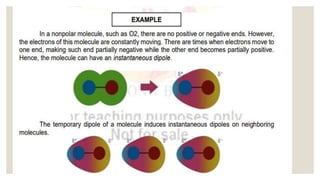
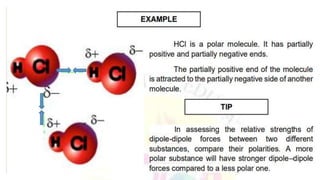
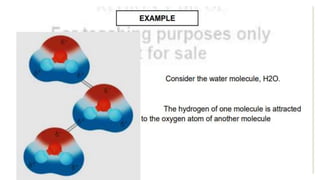
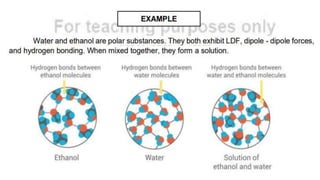
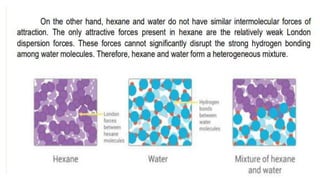
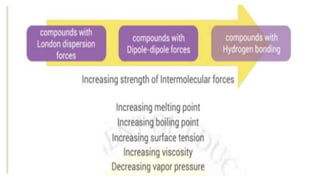
Intermolecular forces are the attractive forces between molecules. The main types are London dispersion forces, dipole-dipole forces, and hydrogen bonding. Properties like melting point, boiling point, solubility, and viscosity depend on the strength of intermolecular forces - stronger forces require more energy to overcome and result in higher melting and boiling points, like dissolving like, and slower flow. Hydrogen bonding is the strongest intermolecular force.