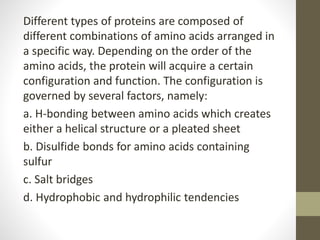
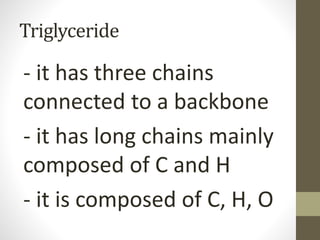
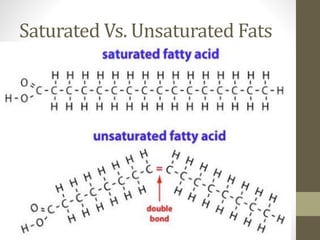
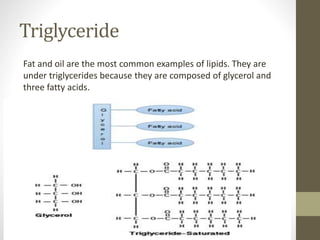
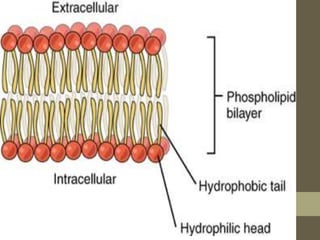
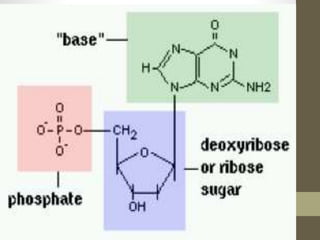
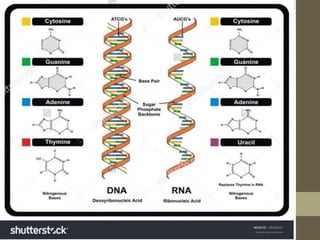
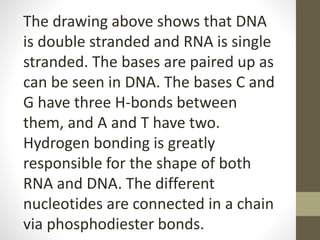
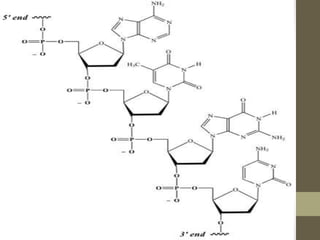
This document provides an overview of the four main types of biological macromolecules: carbohydrates, proteins, lipids, and nucleic acids. It describes the general characteristics and structures of carbohydrates including monosaccharides like glucose, disaccharides, and polysaccharides. For proteins, it discusses the building blocks of amino acids and different protein structures like keratin, silk, and collagen. For lipids, it defines triglycerides, phospholipids, fatty acids and cell membranes. The document aims to distinguish between each macromolecule and relate their structures to properties.