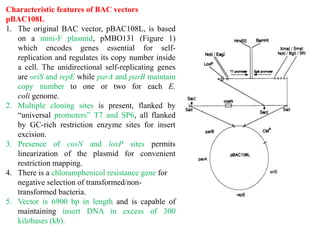
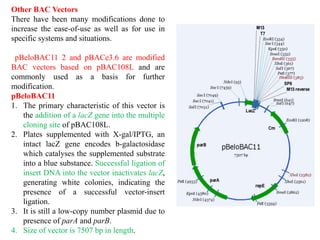
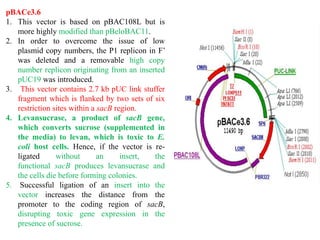
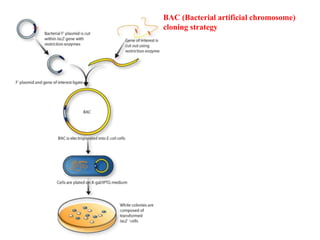
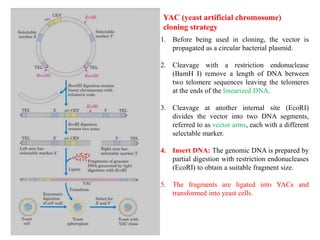
Bacterial artificial chromosomes (BACs) and yeast artificial chromosomes (YACs) are vectors used for cloning large fragments of DNA. BACs can hold inserts up to 350kb and are maintained in bacteria as low copy plasmids, while YACs can hold inserts up to 200kb and are maintained in yeast as artificial chromosomes. Both systems allow cloning and study of large genomic regions and were important tools for early genome projects.