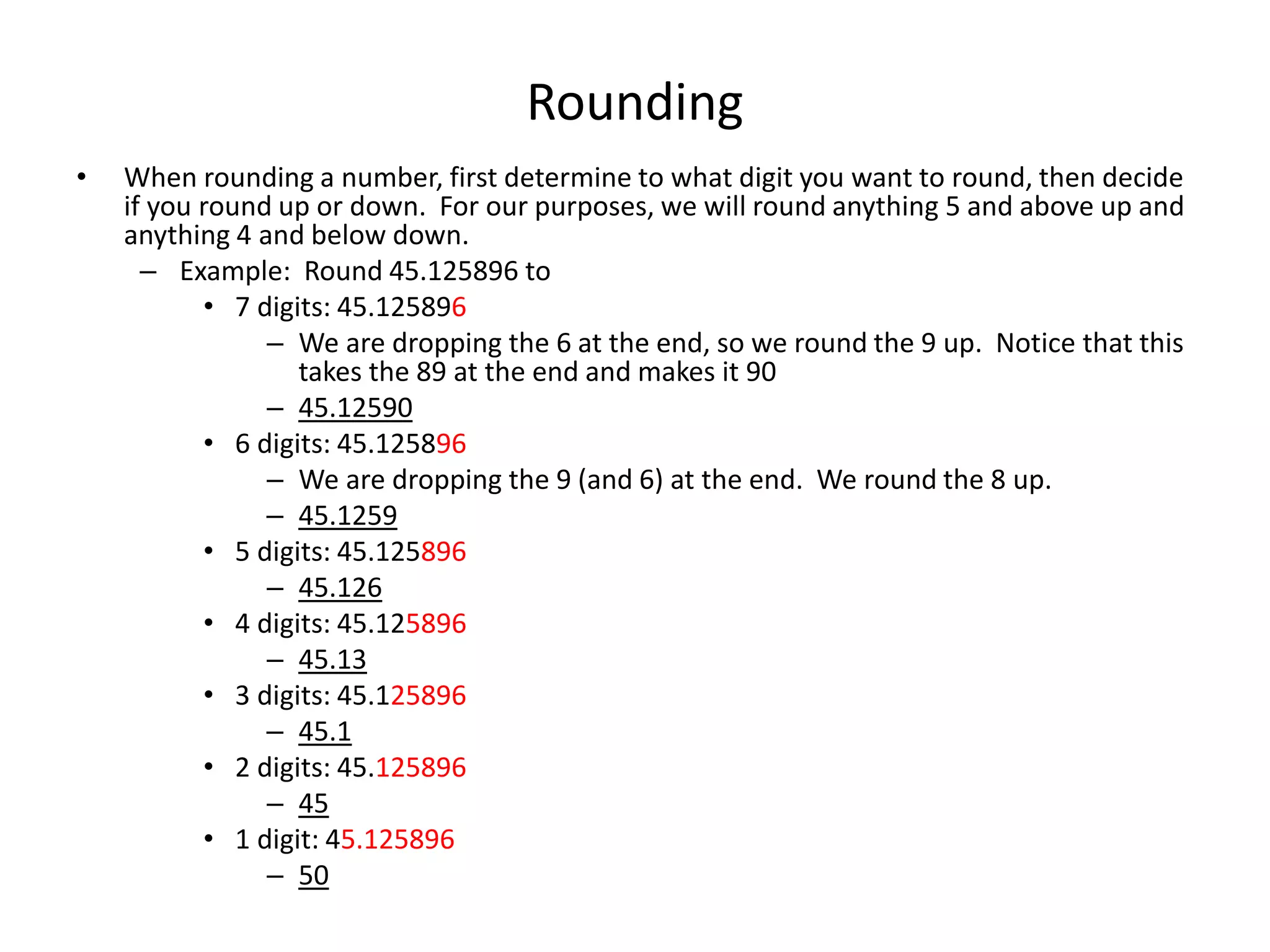
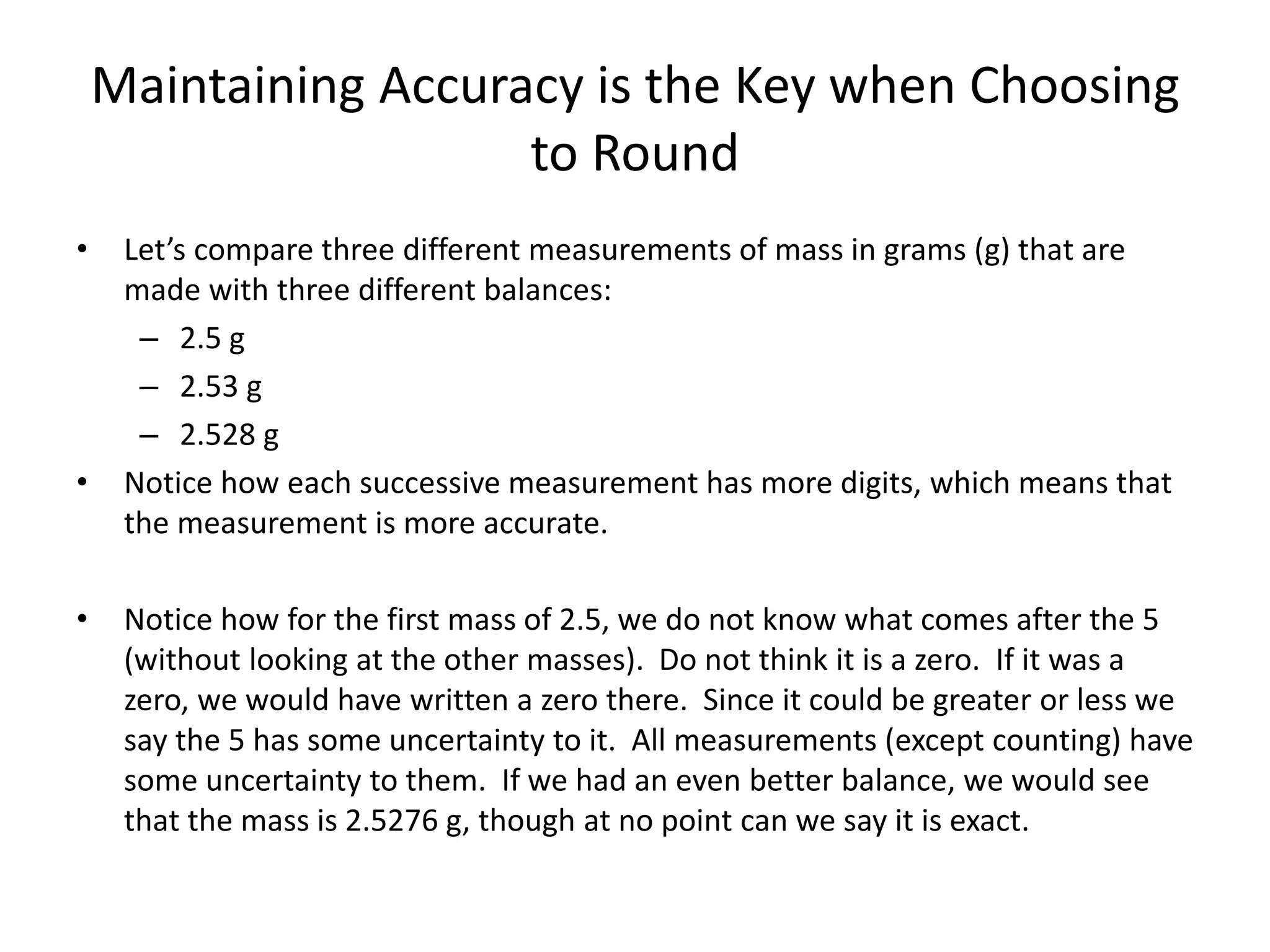
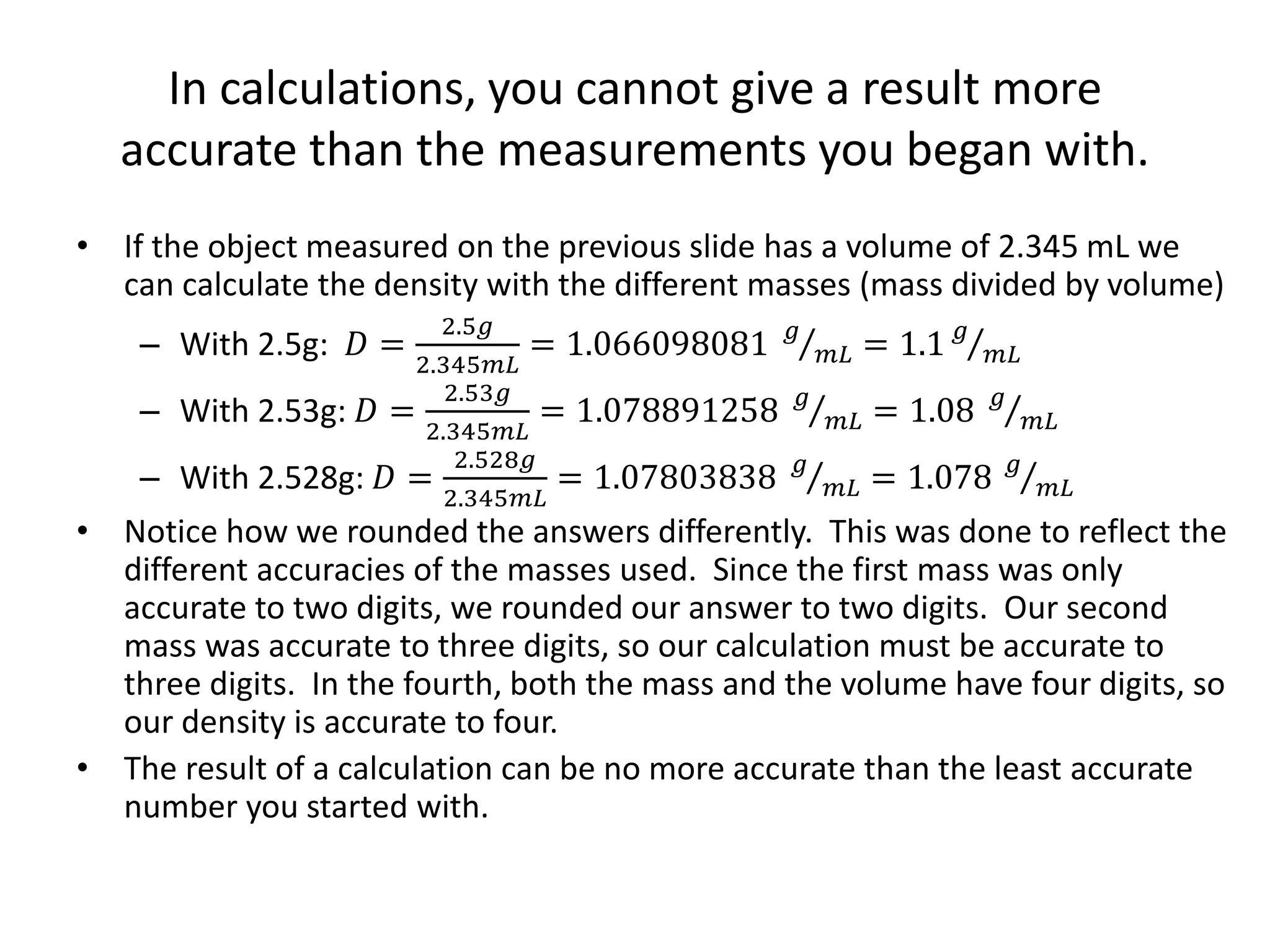
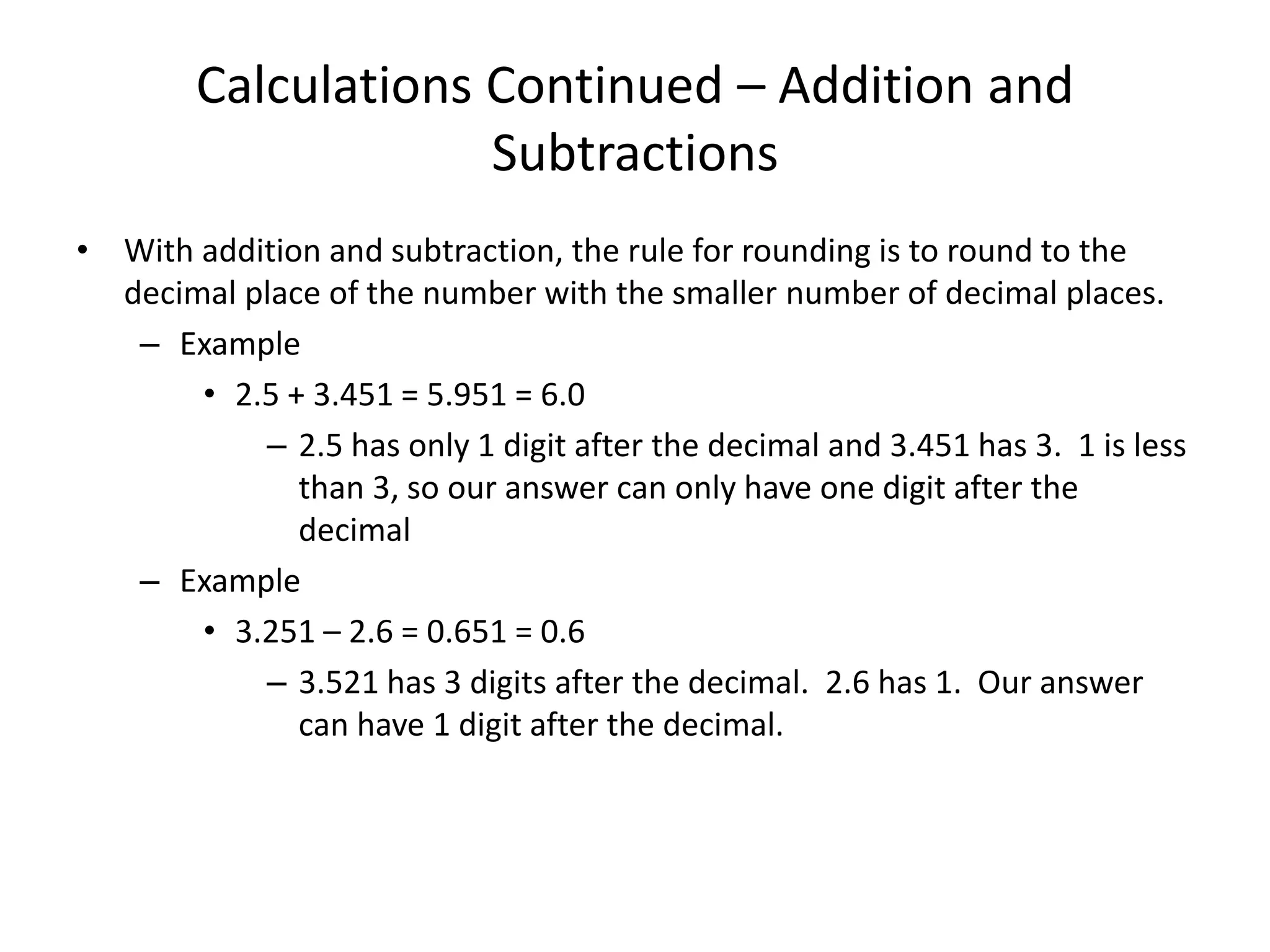
This document discusses significant digits and proper rounding techniques in calculations. It begins by explaining how to round numbers to various places and the rules for determining significant digits. It emphasizes that the accuracy of a calculation is limited by the least accurate initial value. For addition and subtraction, the answer should be rounded to the least number of decimal places. For multiplication and division, the answer should be rounded to the same number of significant digits as the least accurate factor or dividend. Examples are provided to demonstrate rounding calculations correctly according to these rules.