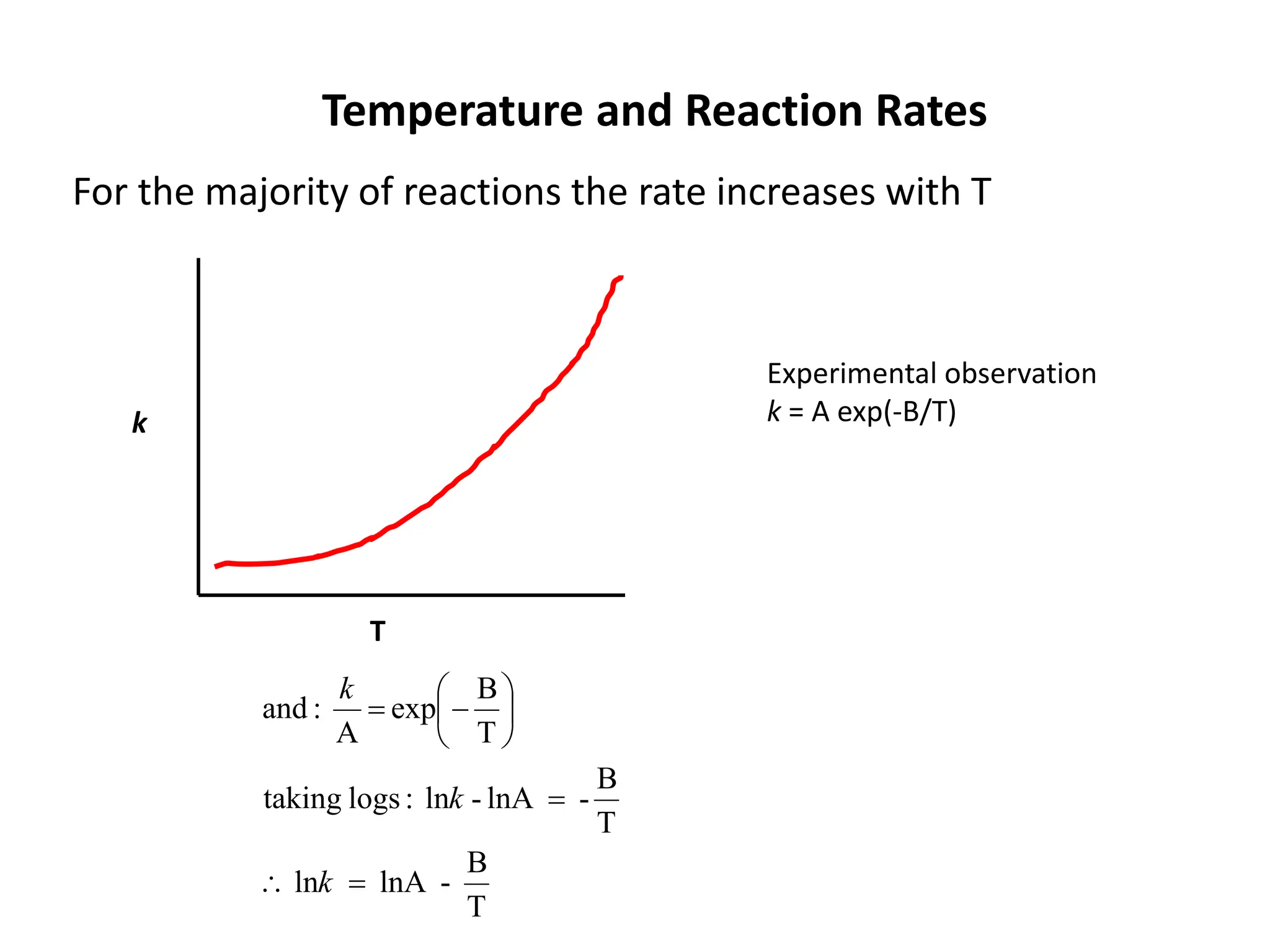
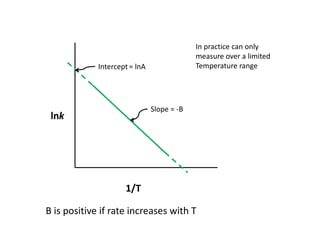
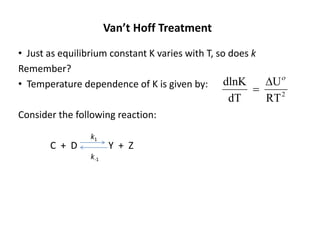
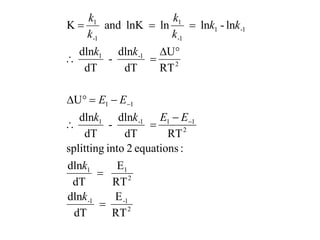
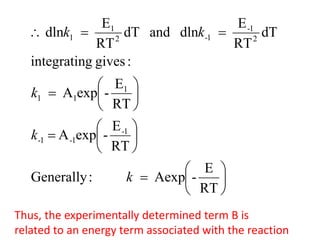
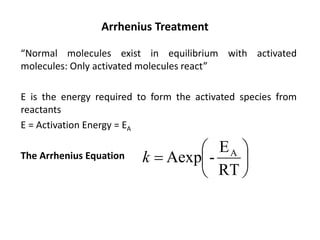
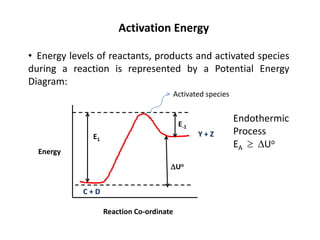
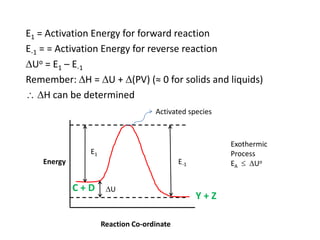
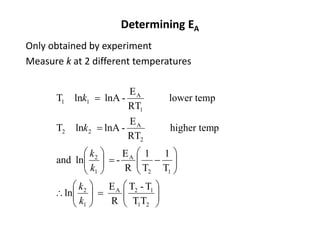
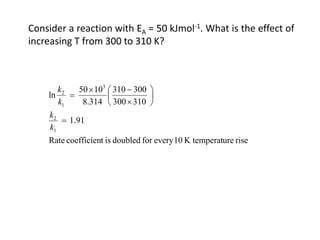
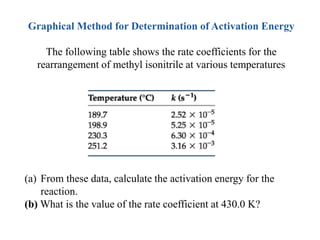
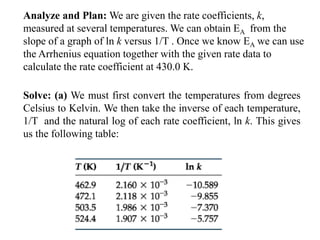
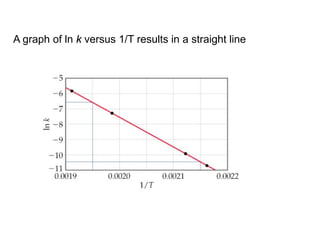
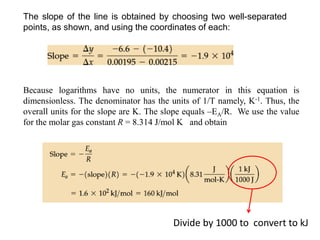
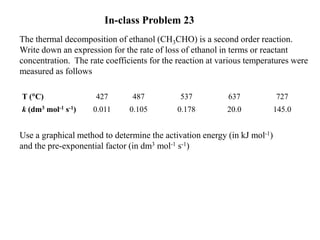
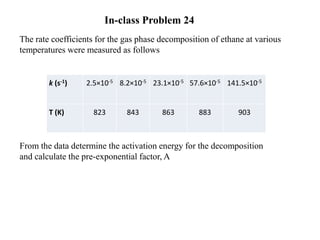
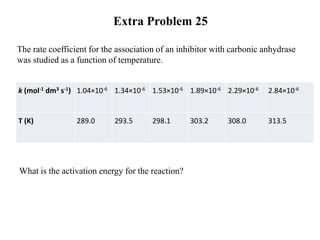
The document discusses the relationship between temperature and reaction rates, referencing the Arrhenius equation and activation energy. It details methods for experimentally determining activation energy and rate coefficients at various temperatures, including graphical methods to analyze data. Examples and problems related to chemical reactions under varying temperatures are also provided to demonstrate these concepts.