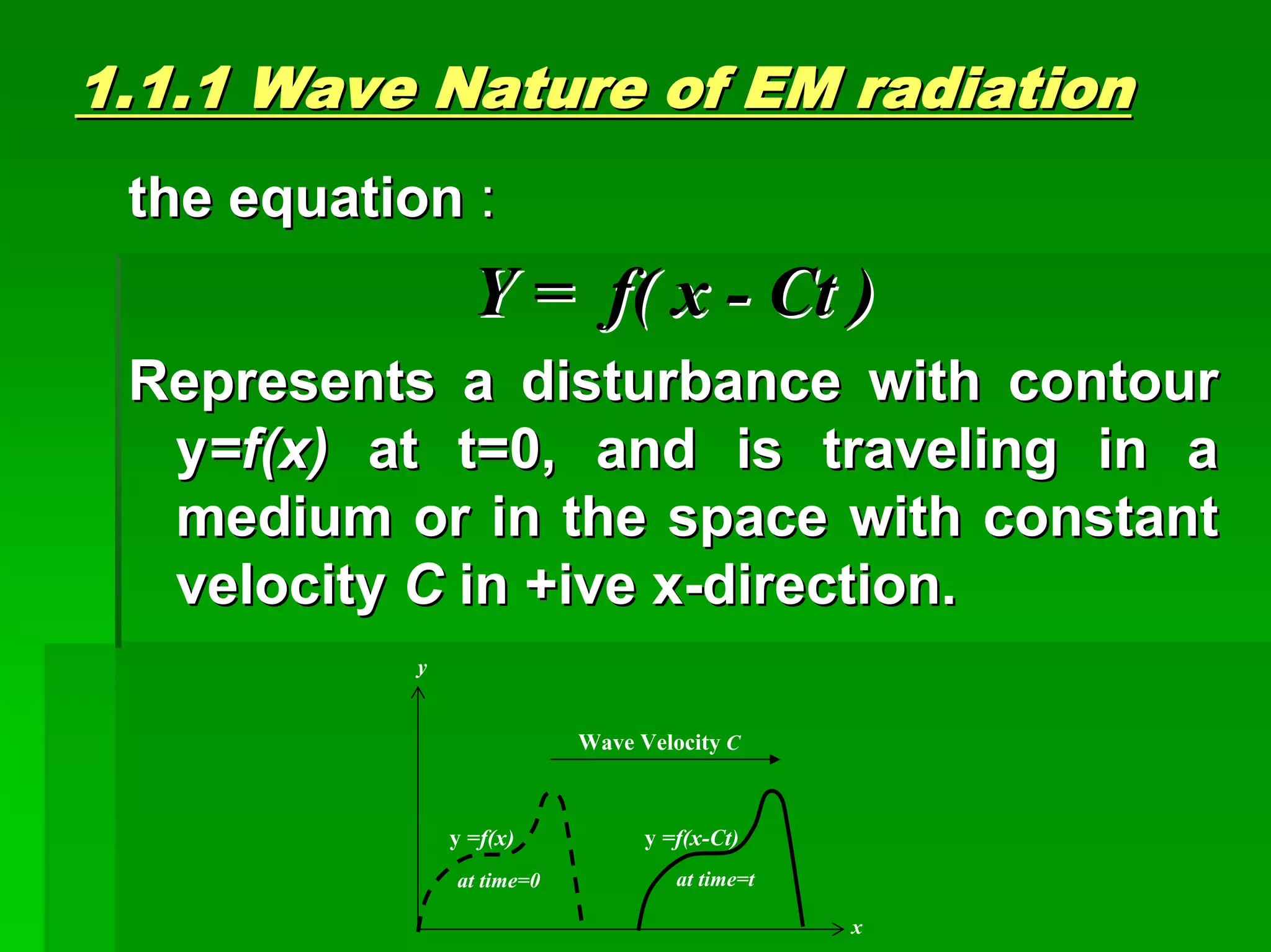
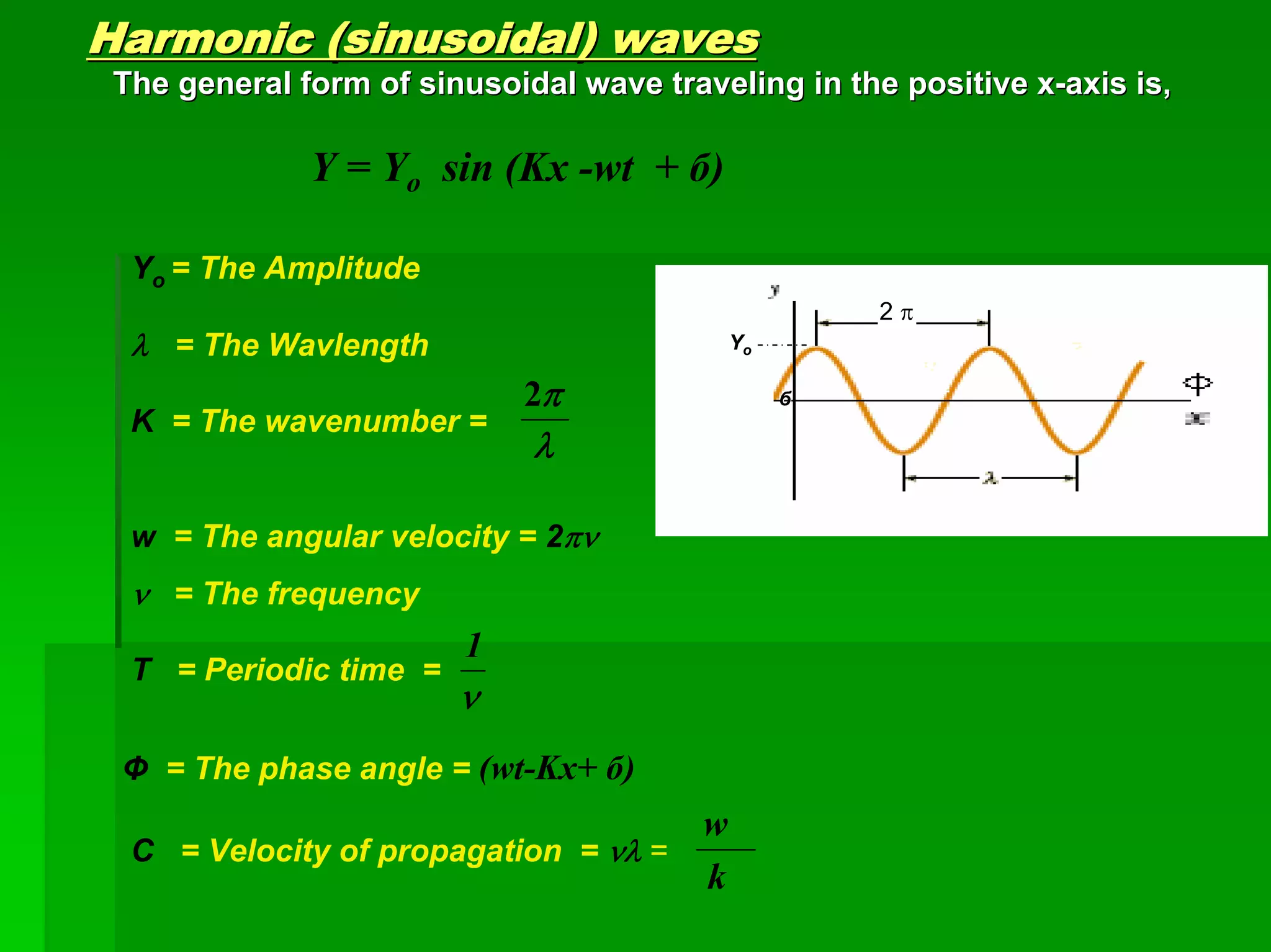
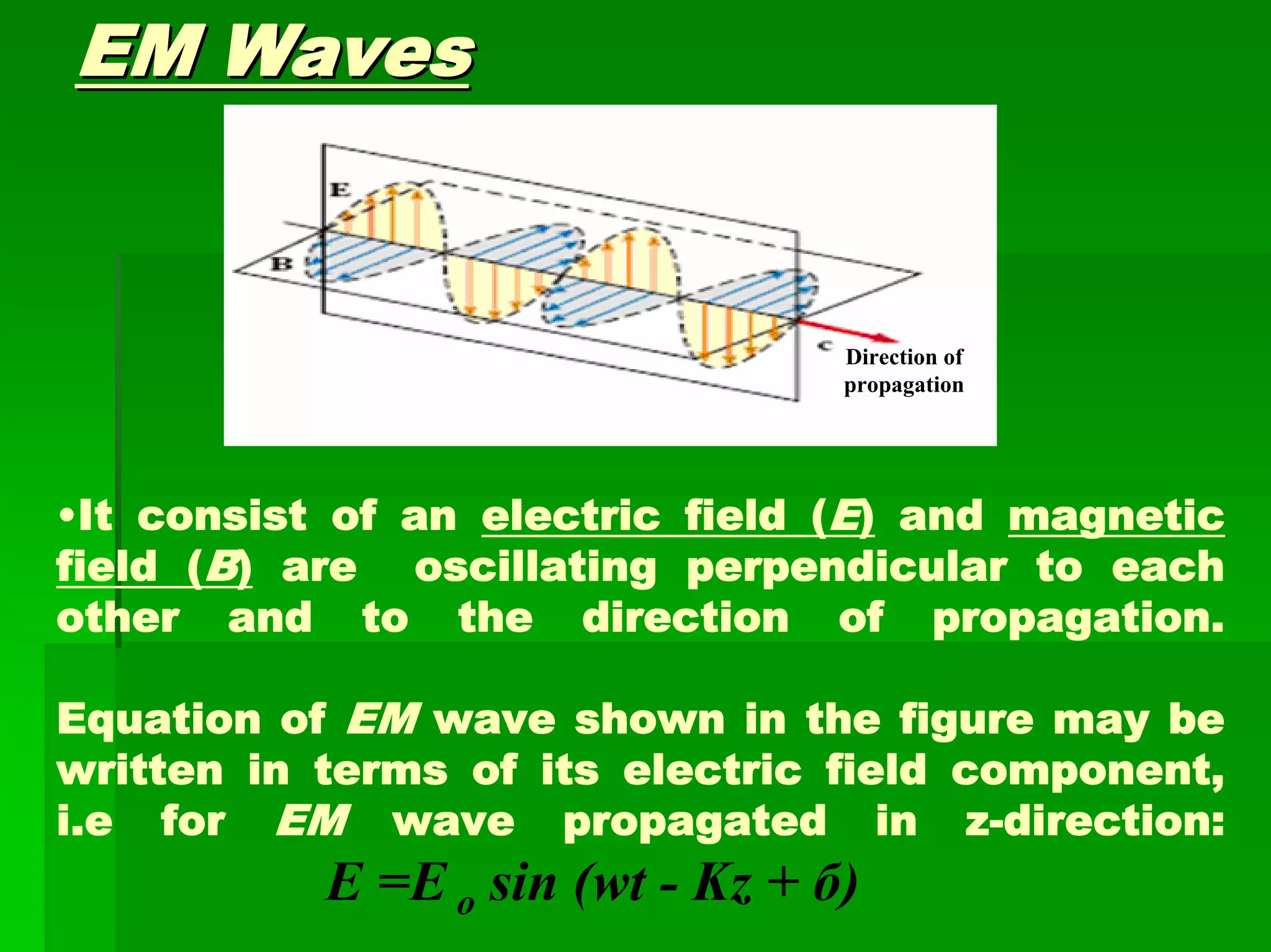
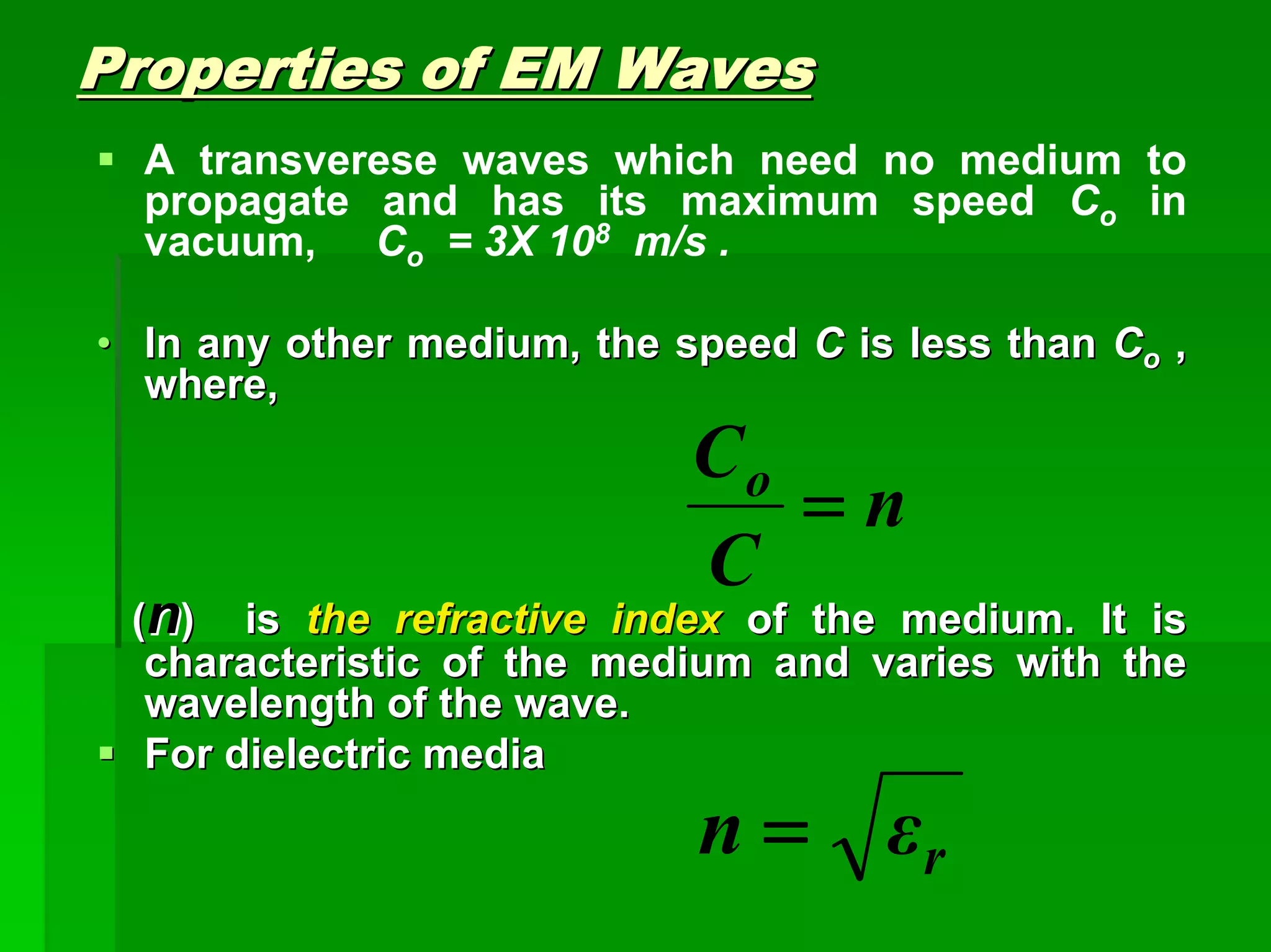
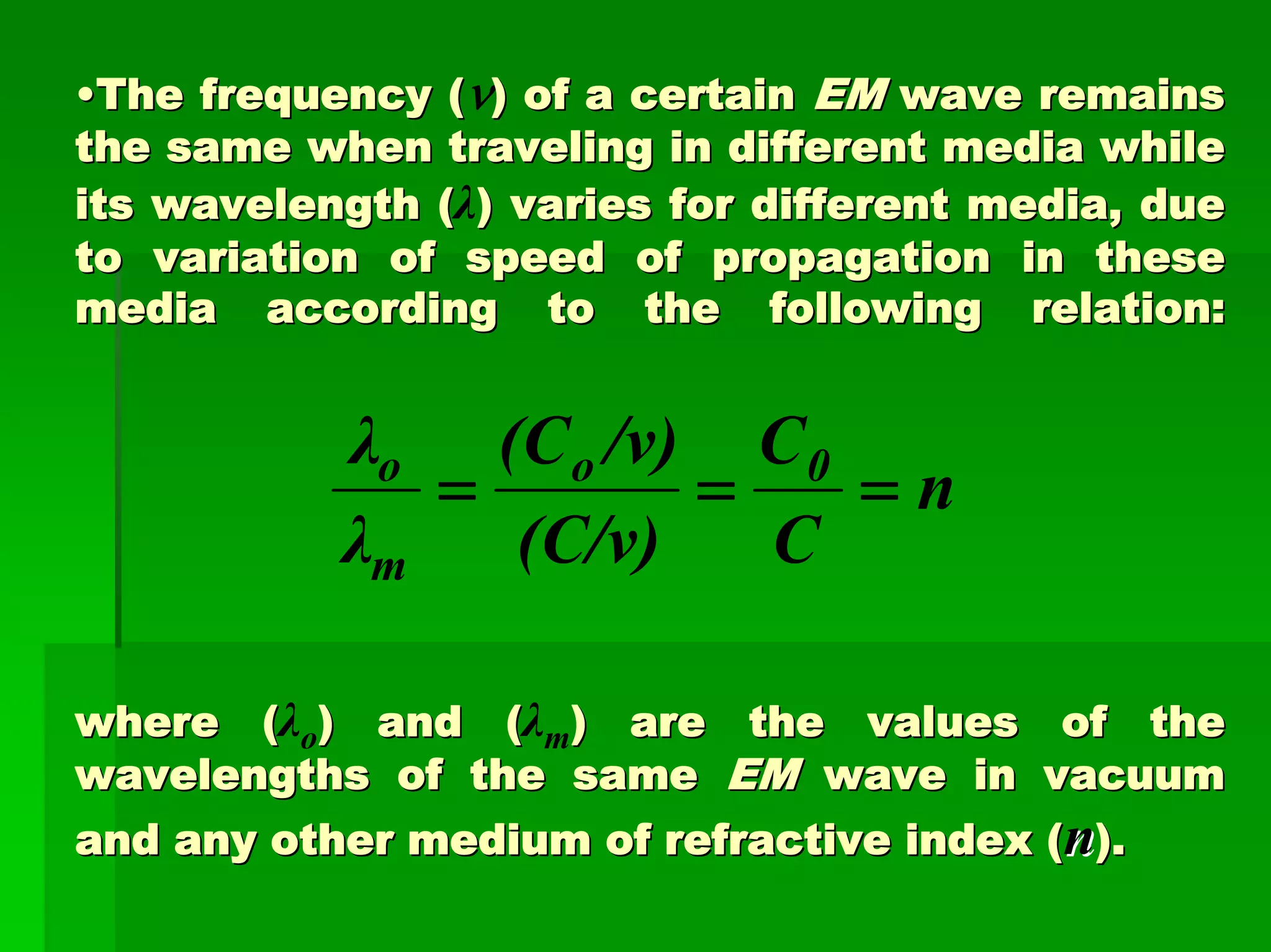
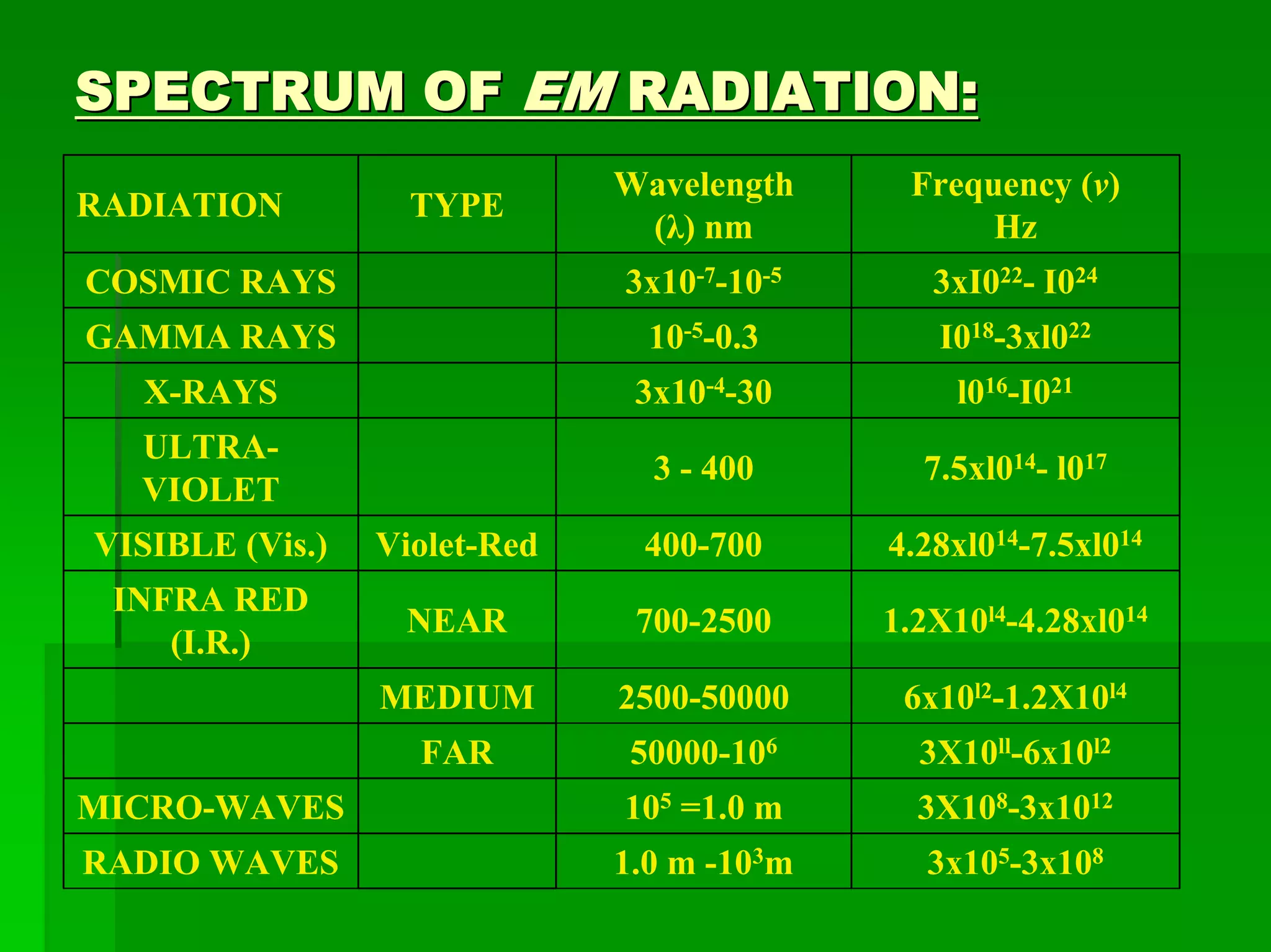
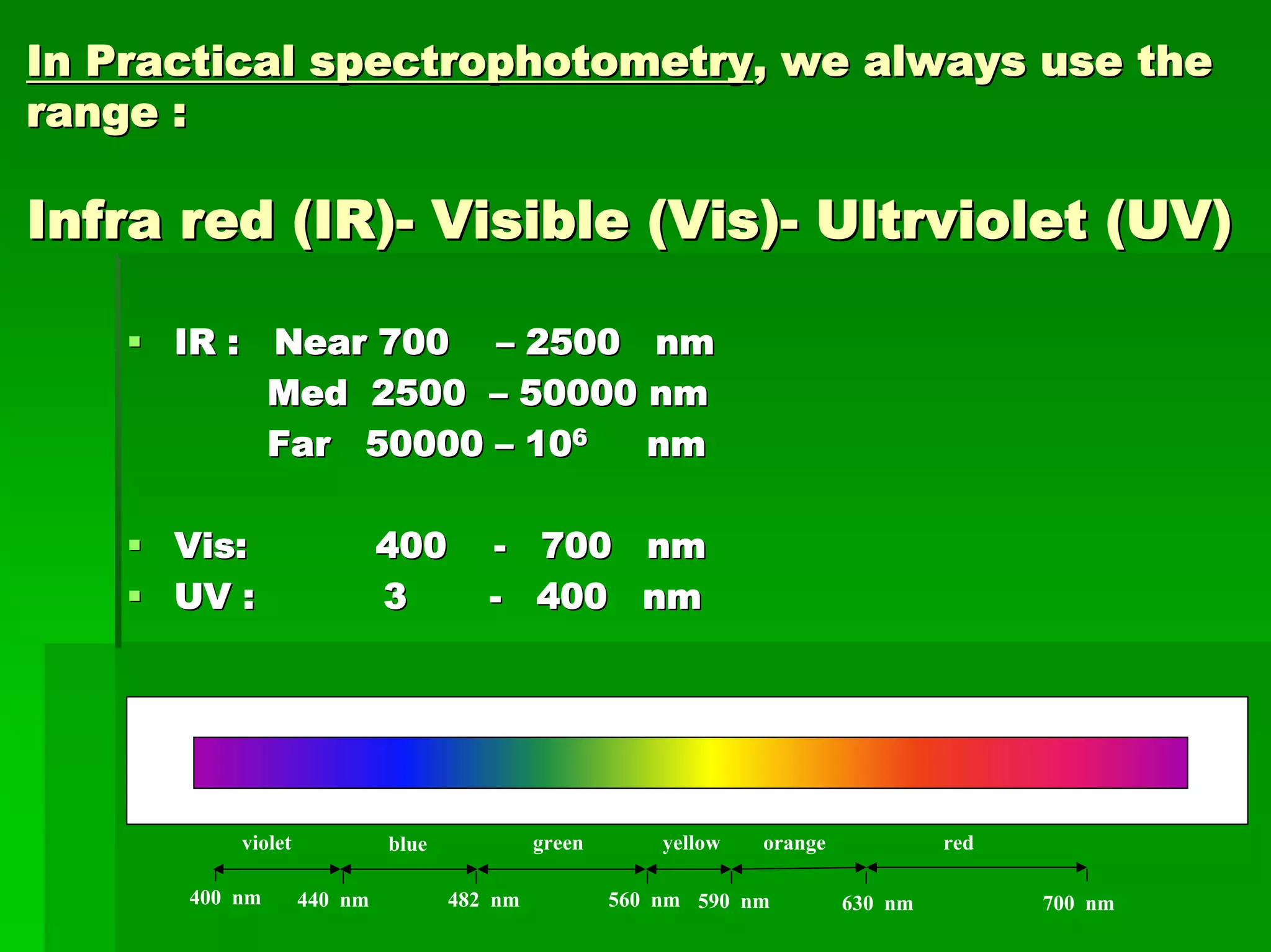
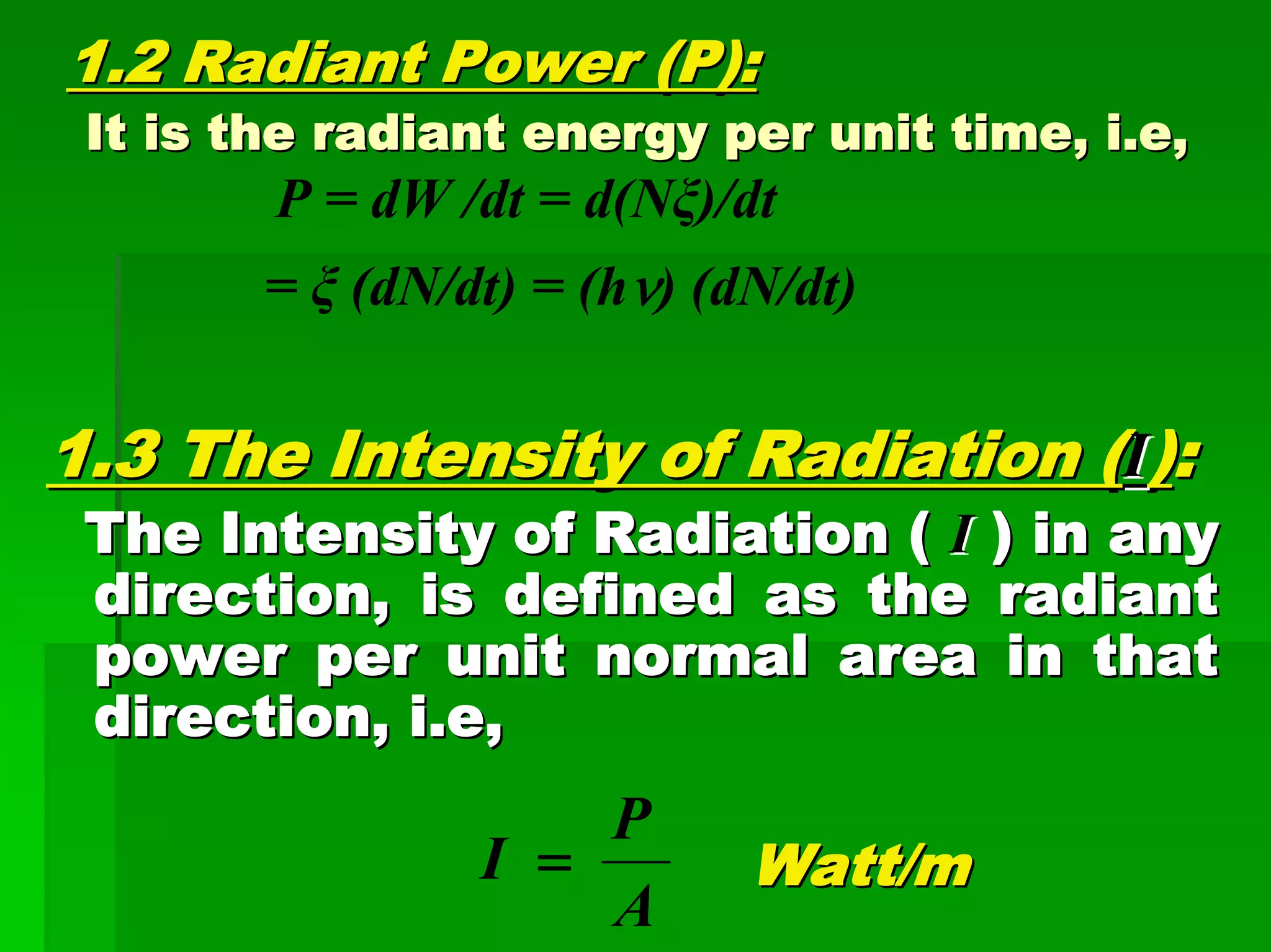
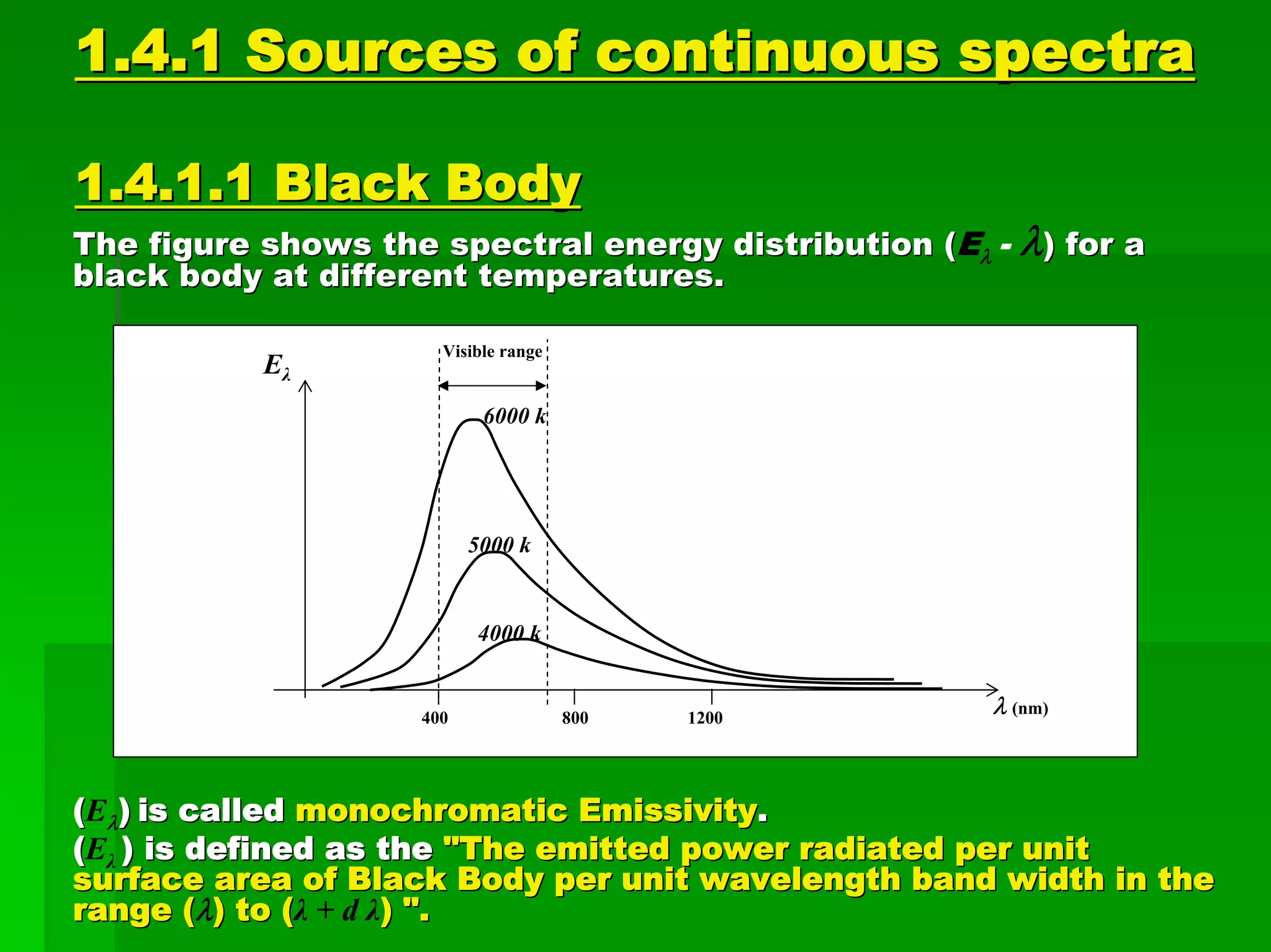
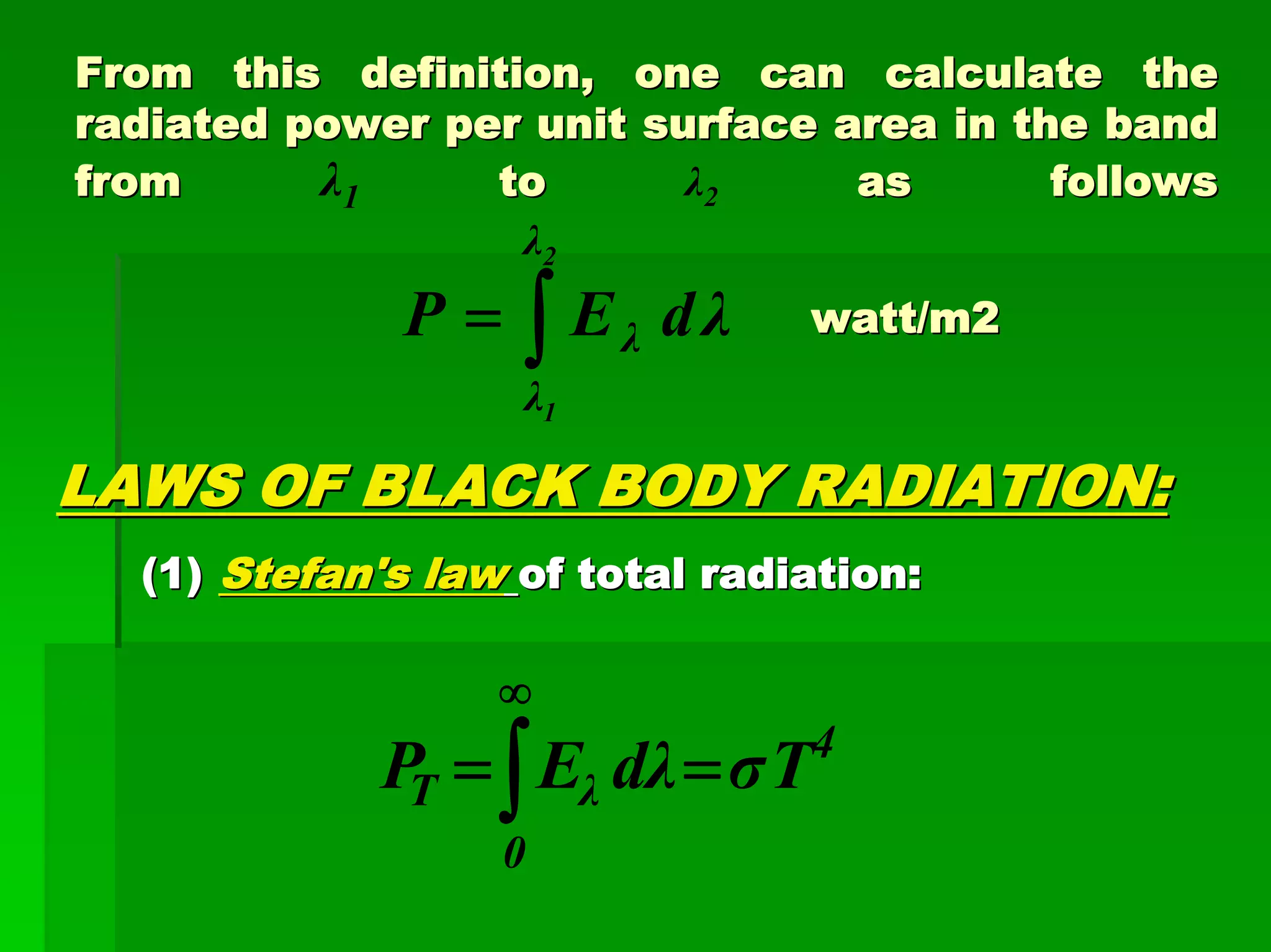
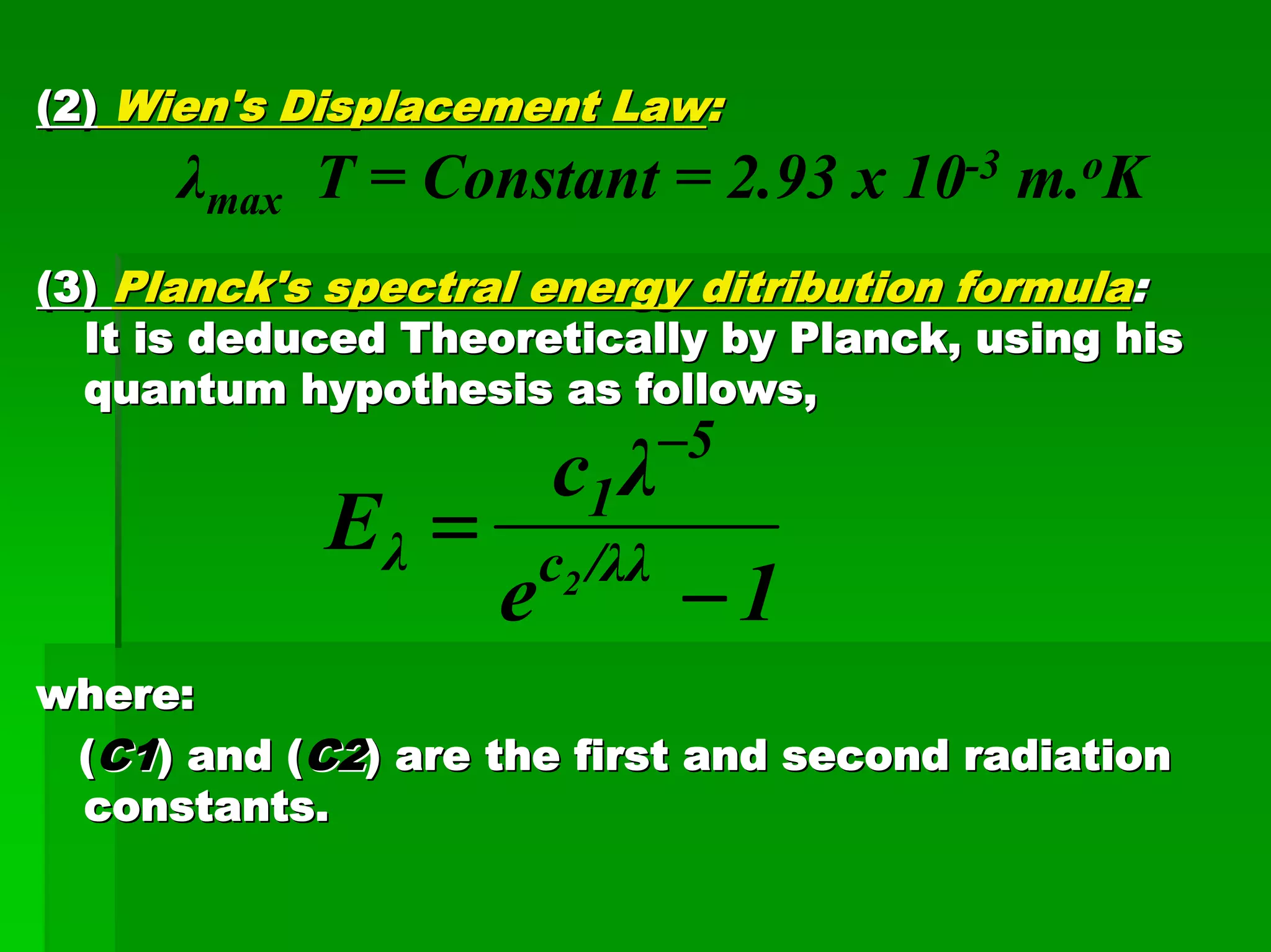
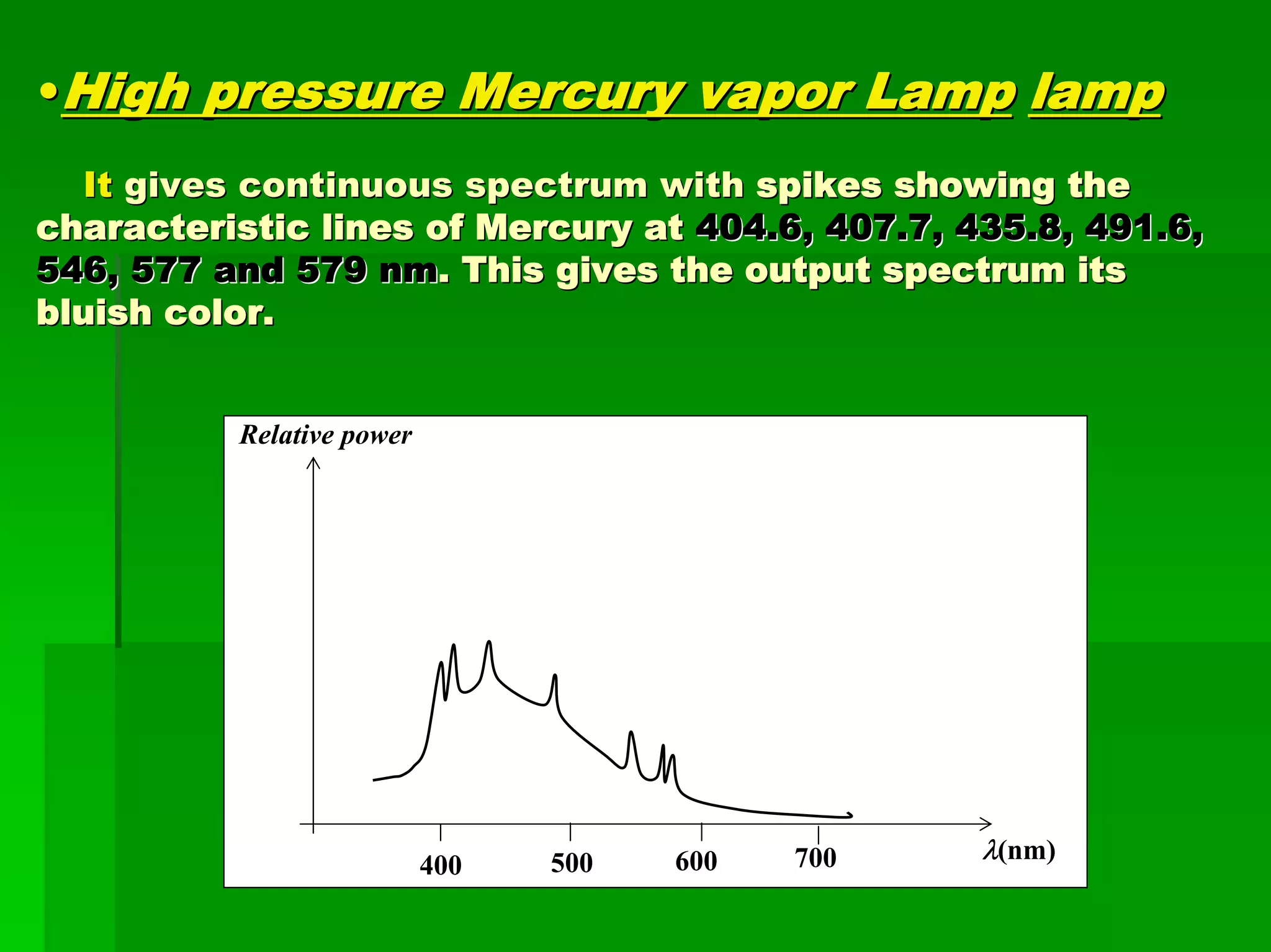
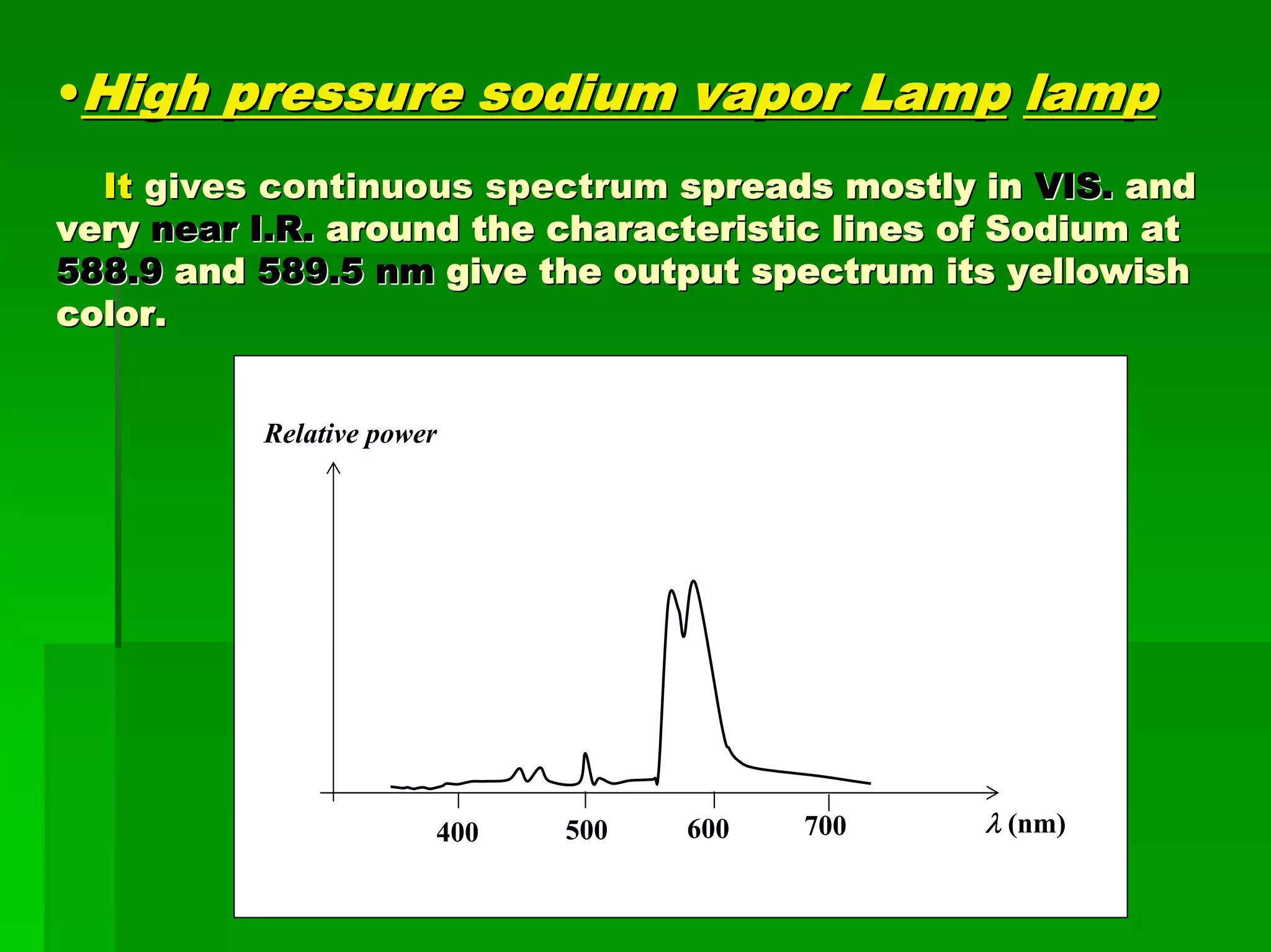
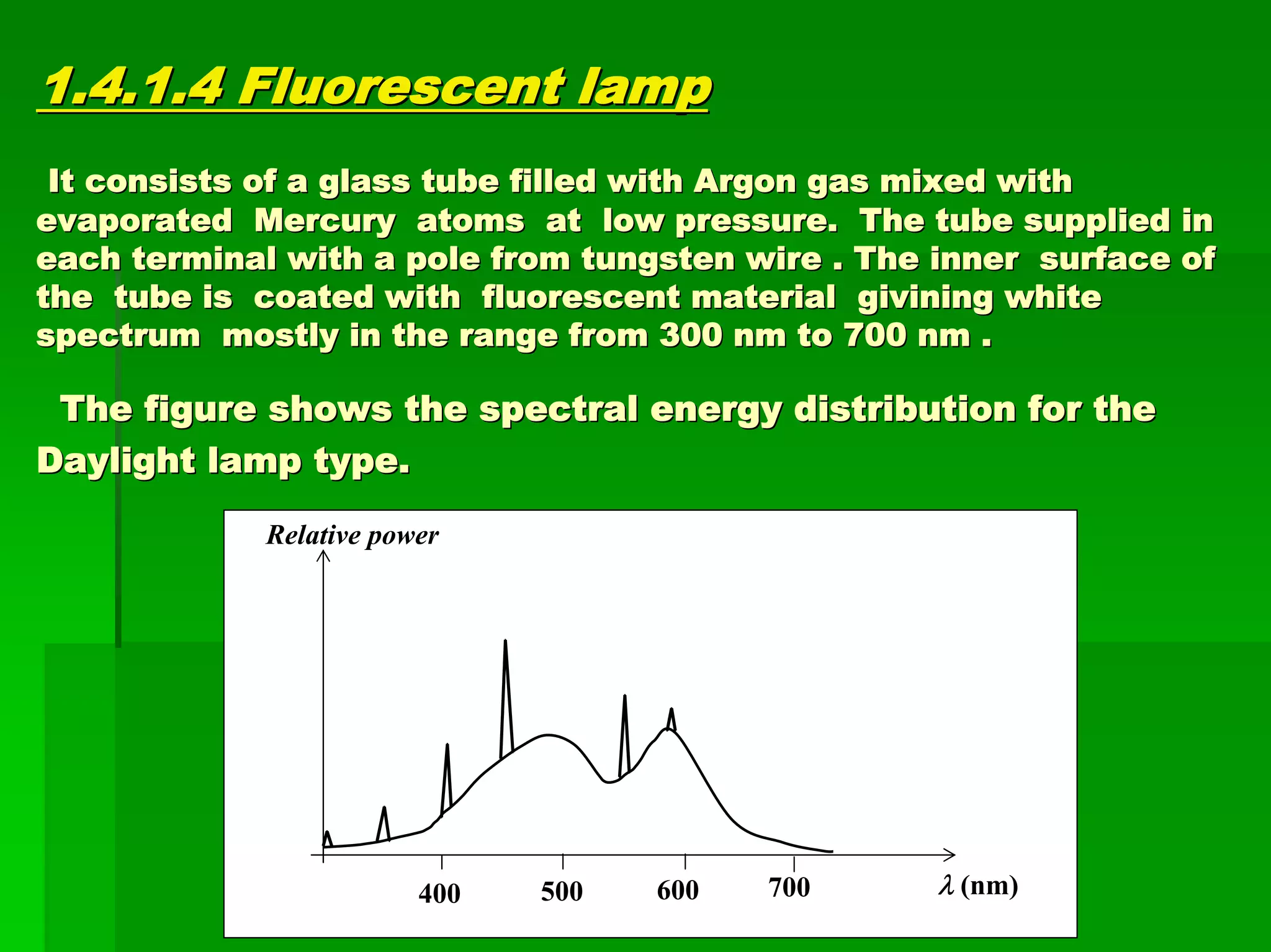
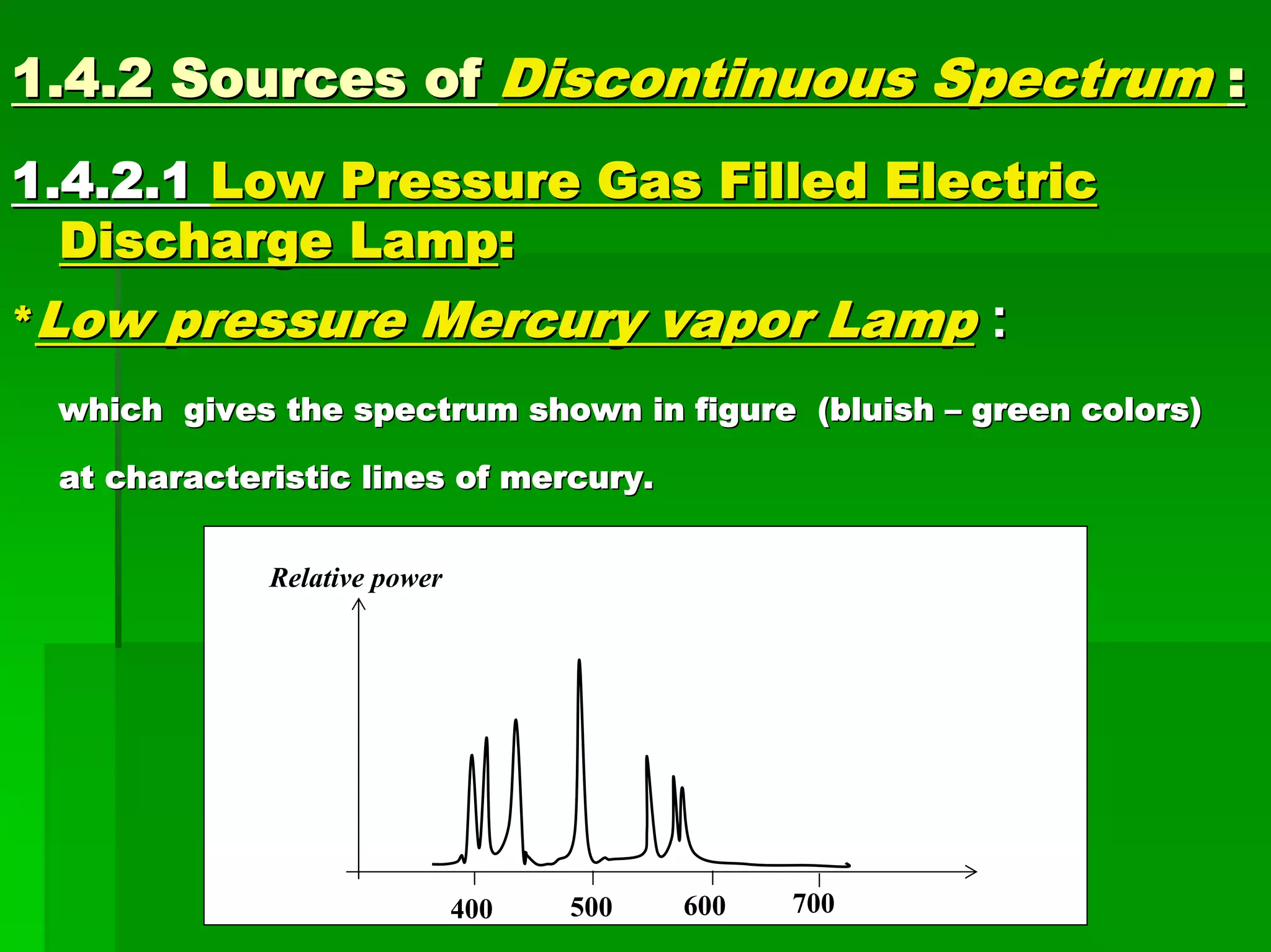
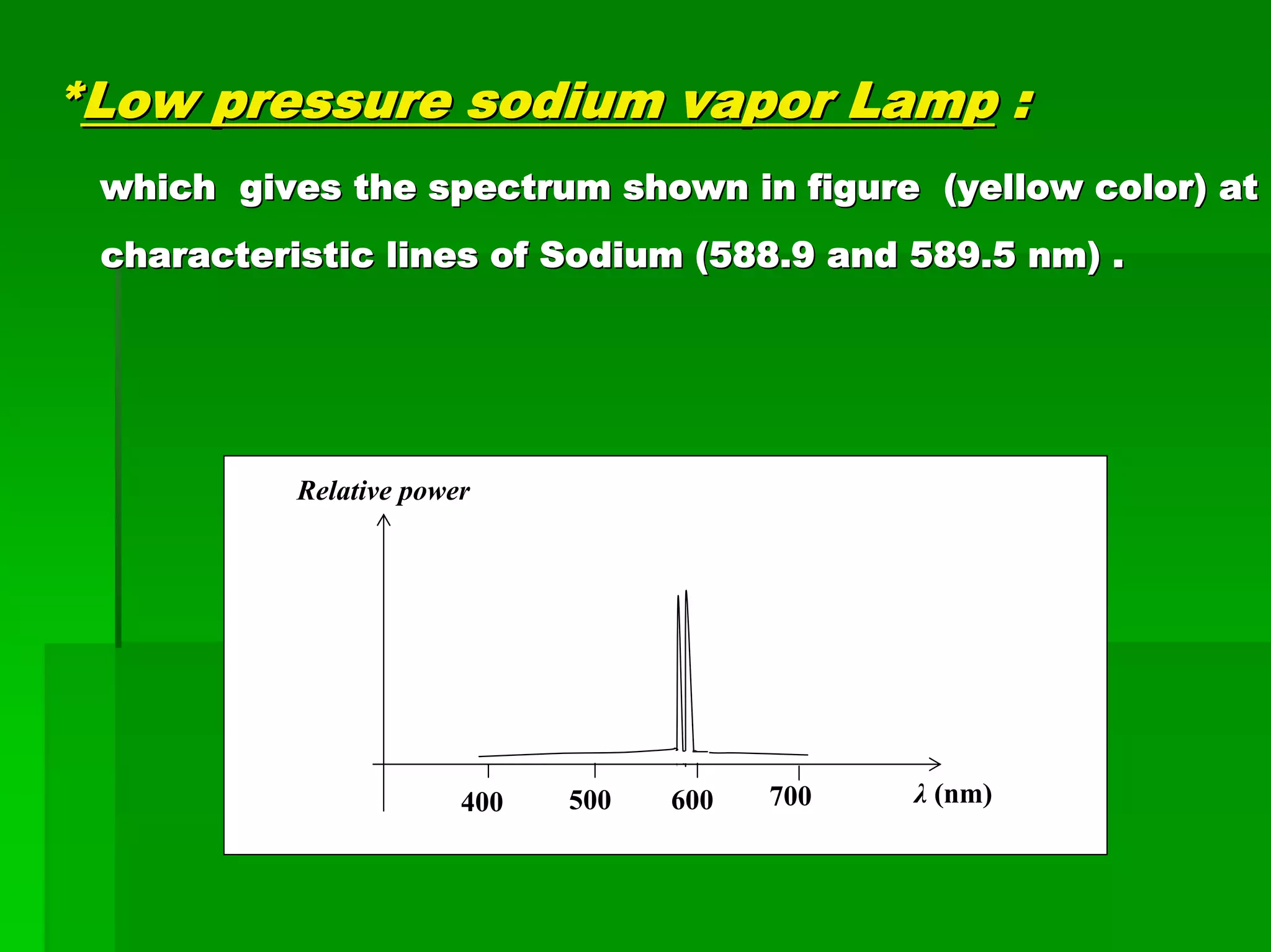
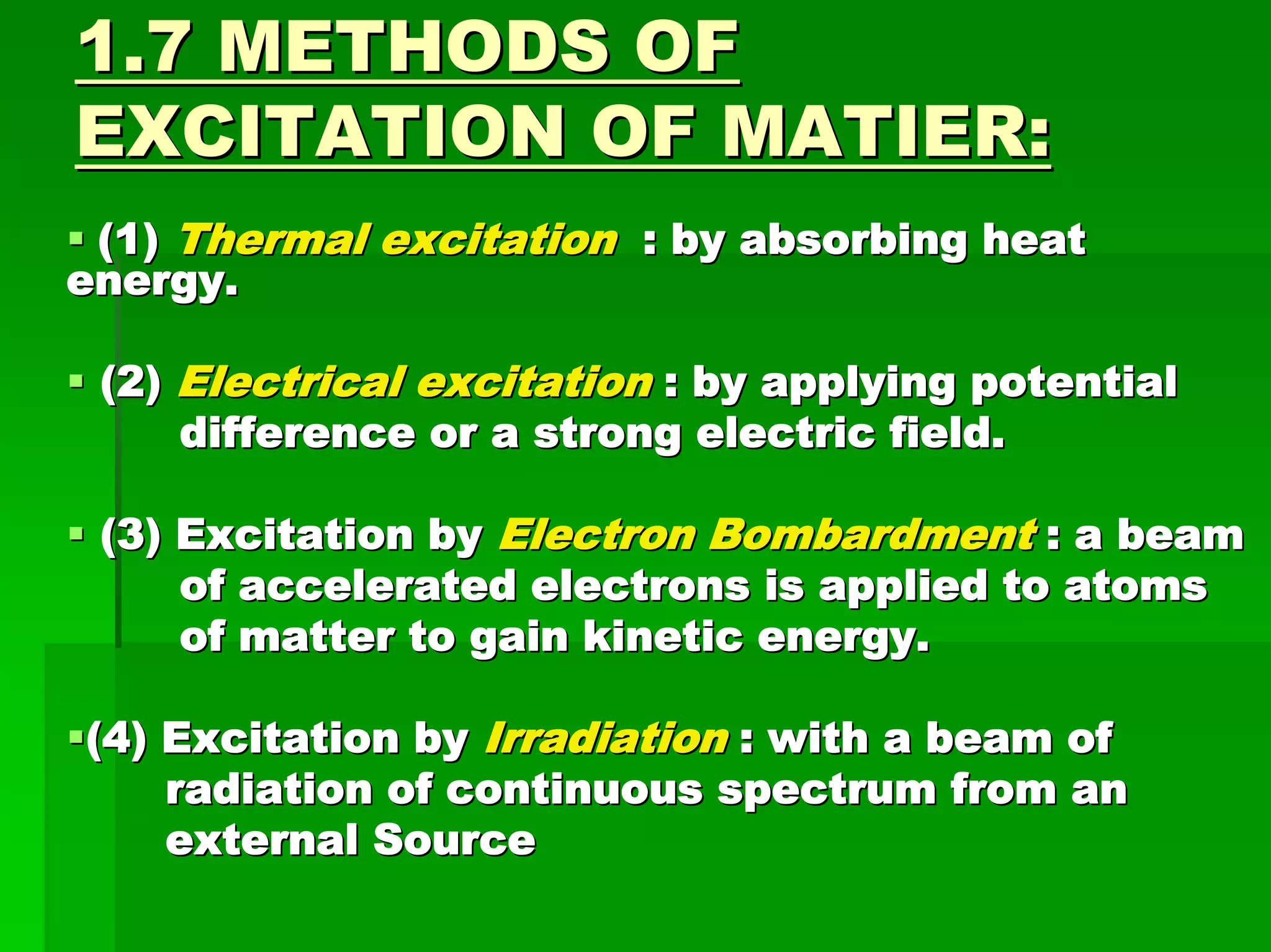
This document discusses optical analytical instrumentation and the interaction of electromagnetic (EM) radiation with matter. It describes different forms of spectra including absorption, emission, fluorescence, polarization, and scattering. A major class of analytical instruments is based on the interaction of radiation with matter, such as spectrophotometers, fluorometers, and polarimeters. The document then covers the nature of EM radiation as both waves and photons, properties of EM waves, sources of radiation including black bodies and gas discharge lamps, and the relationship between radiation, matter, and spectra.