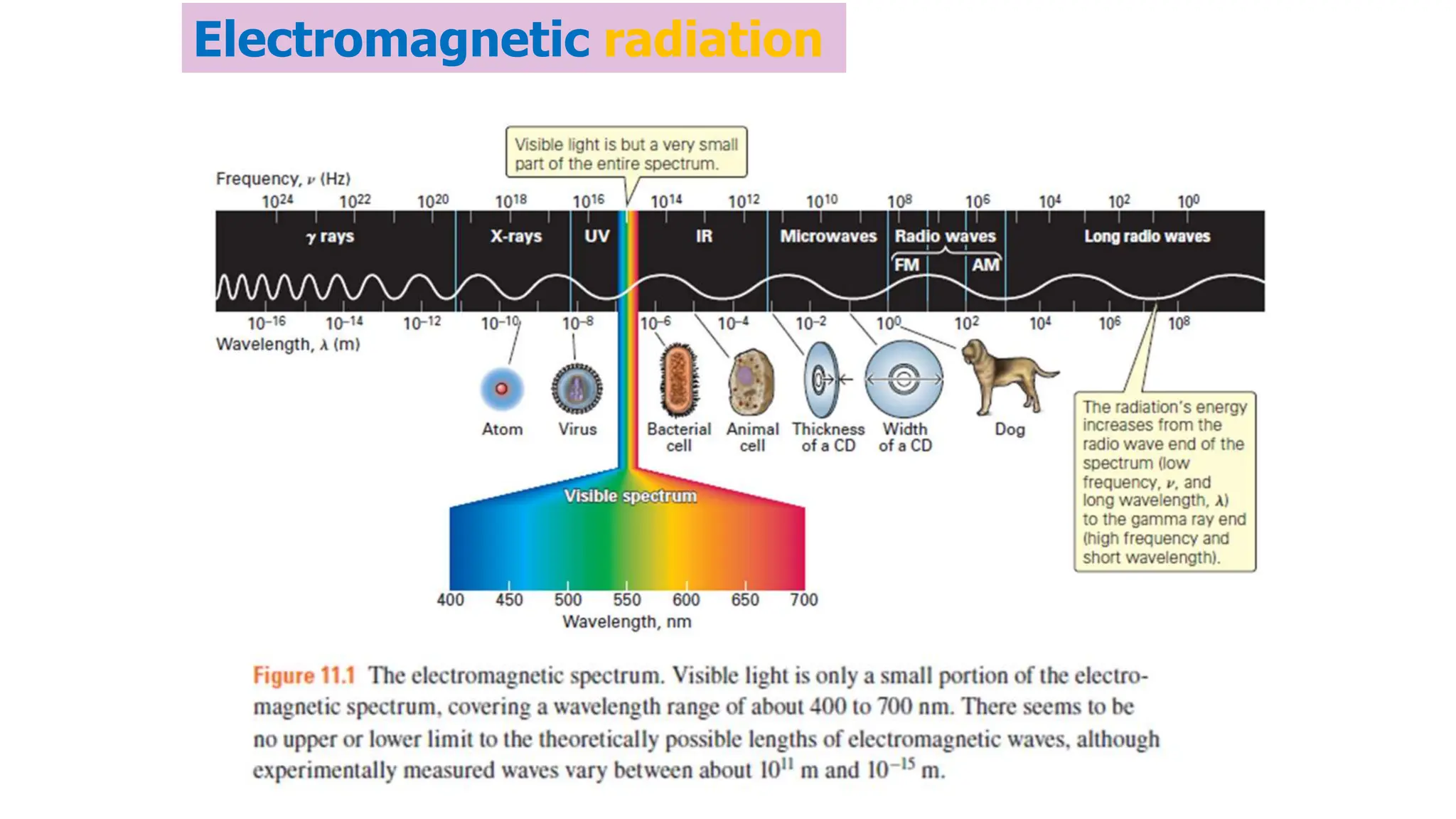
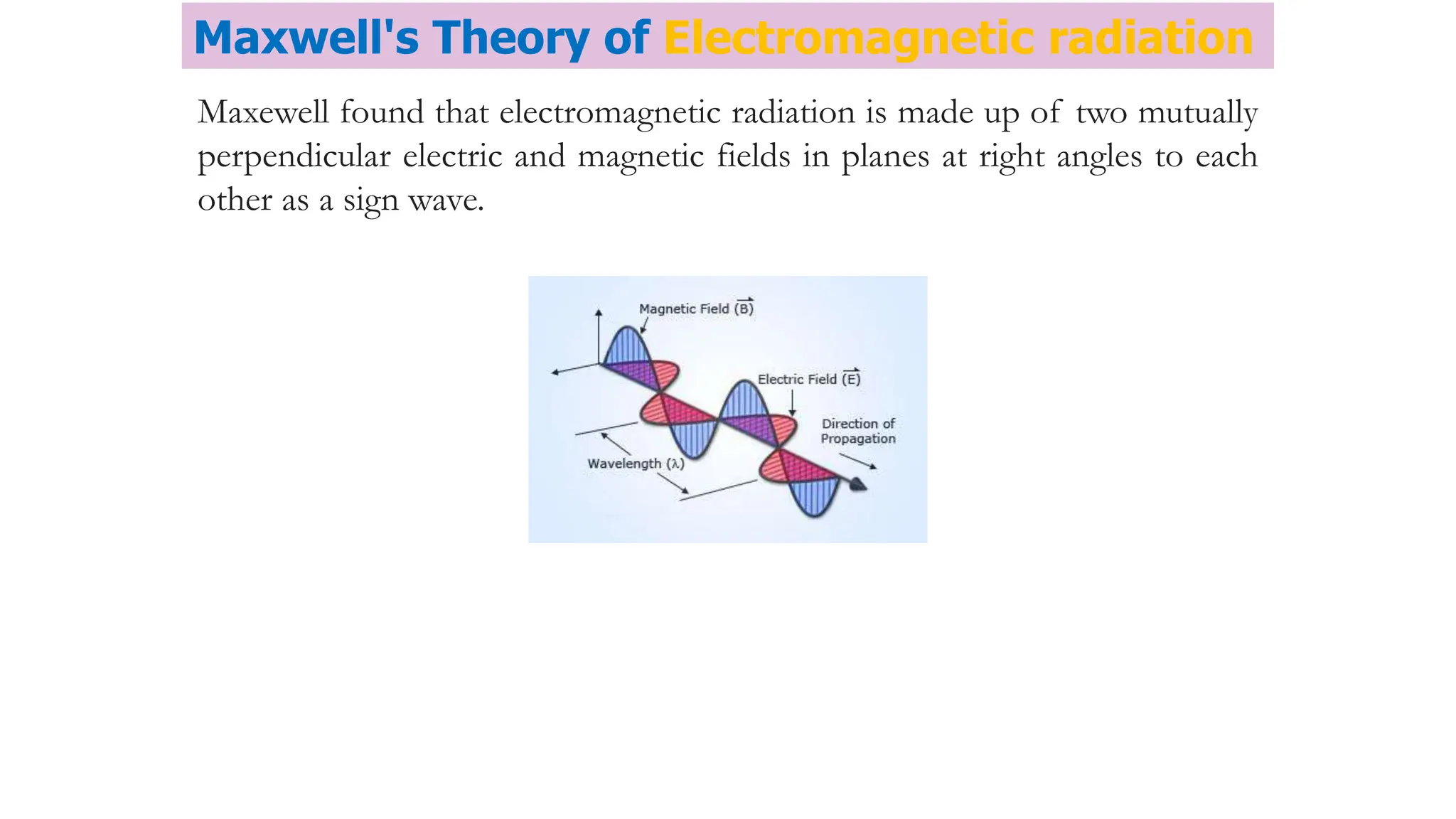
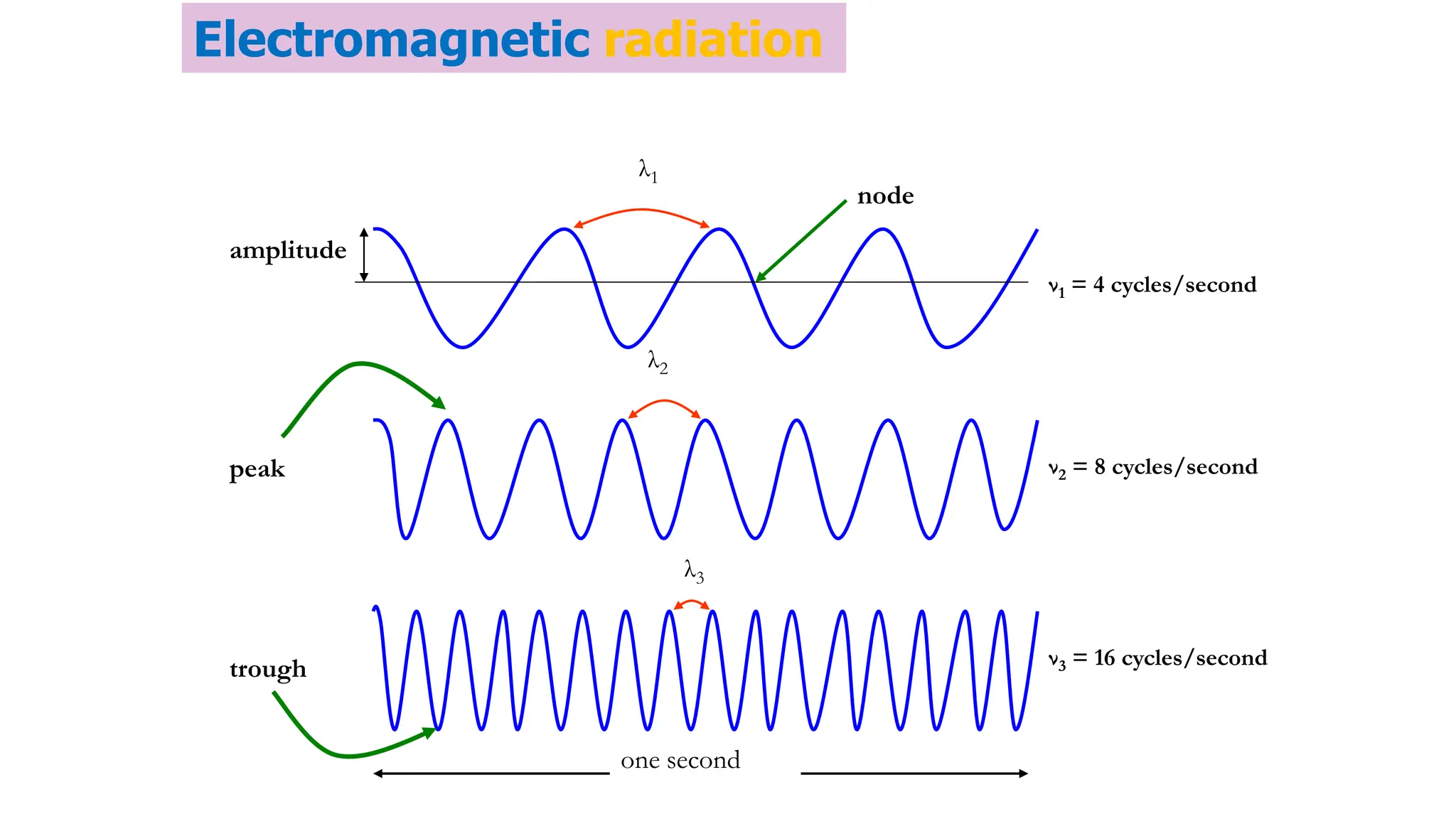
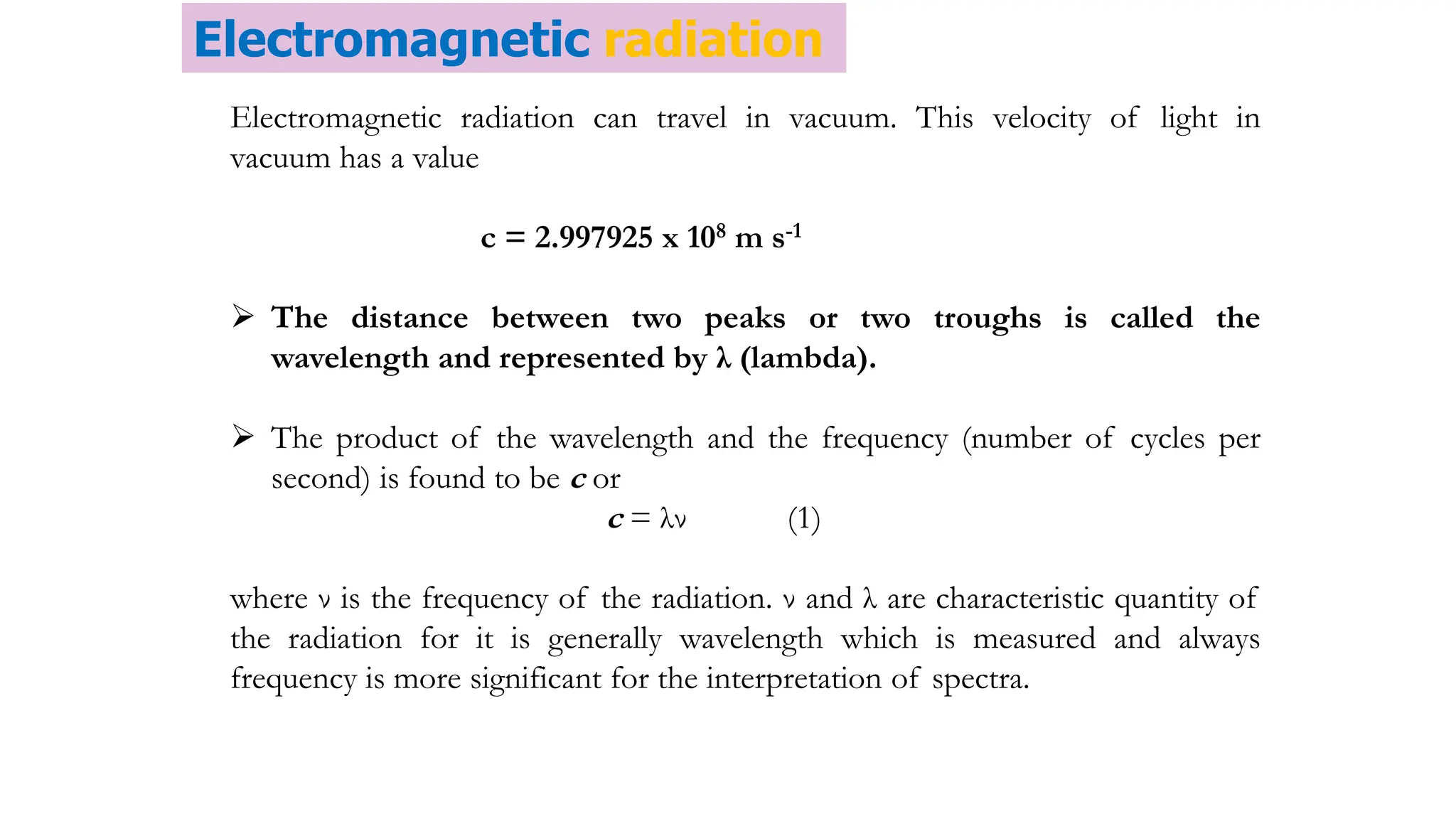
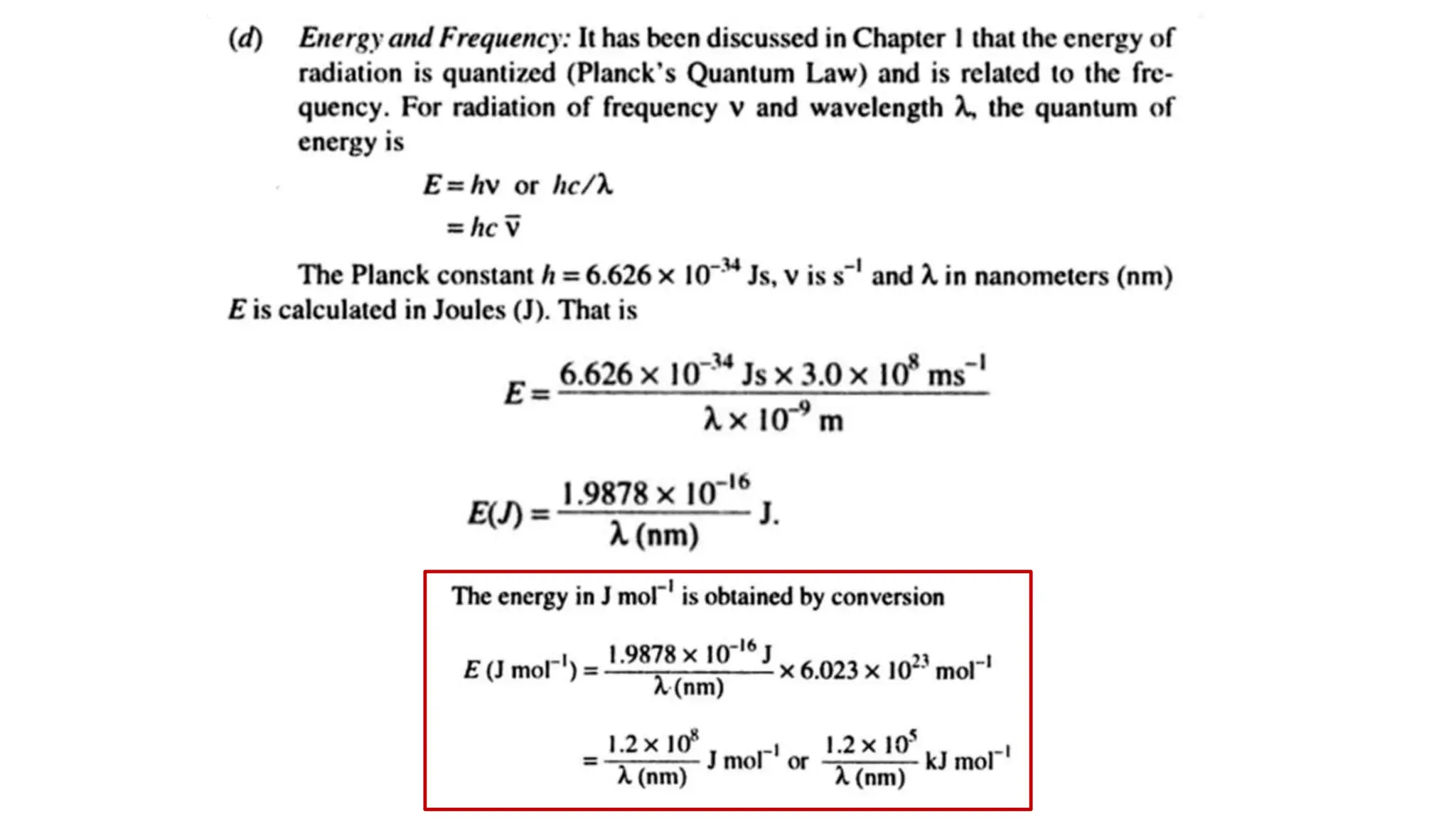
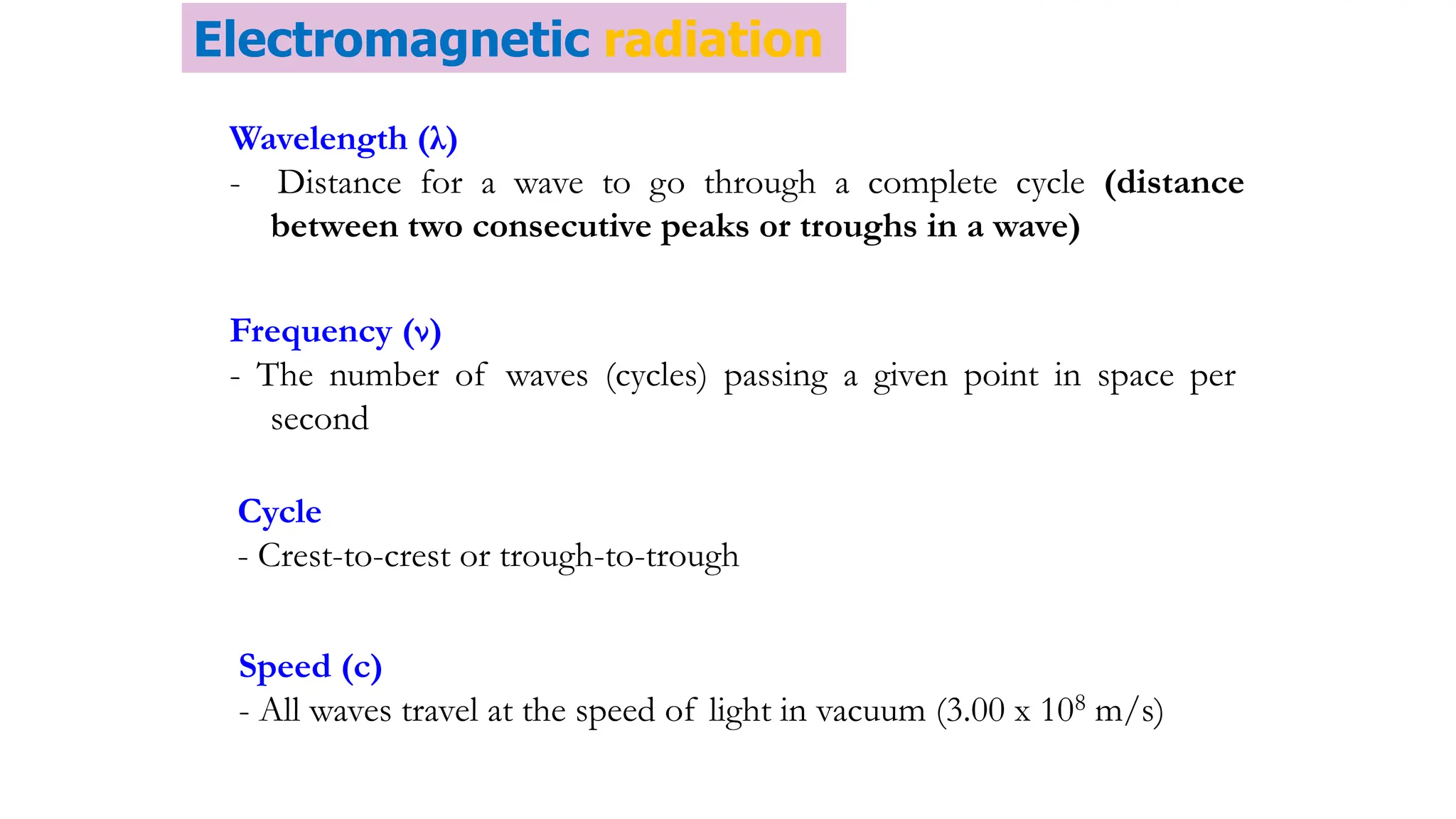
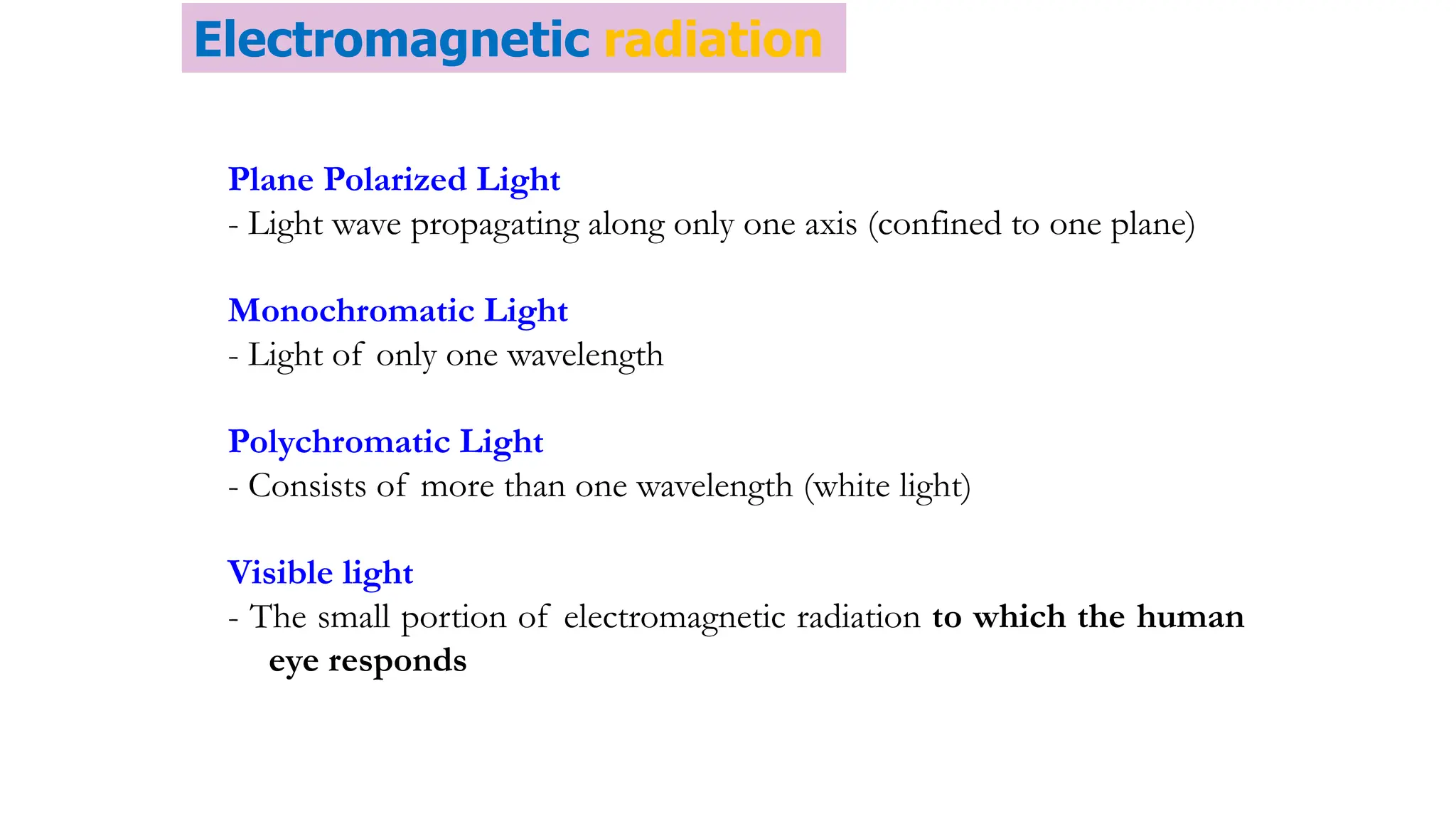
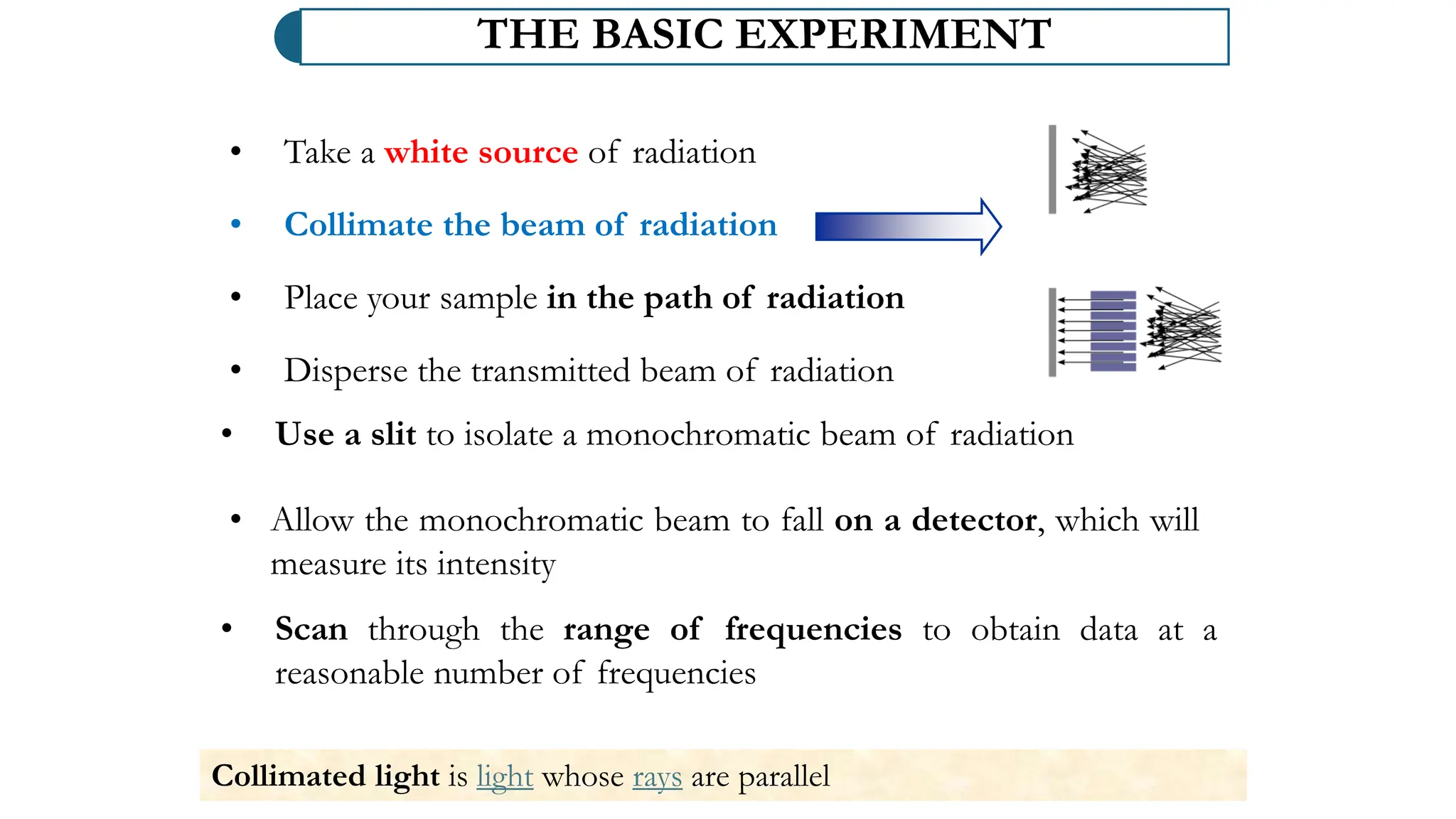
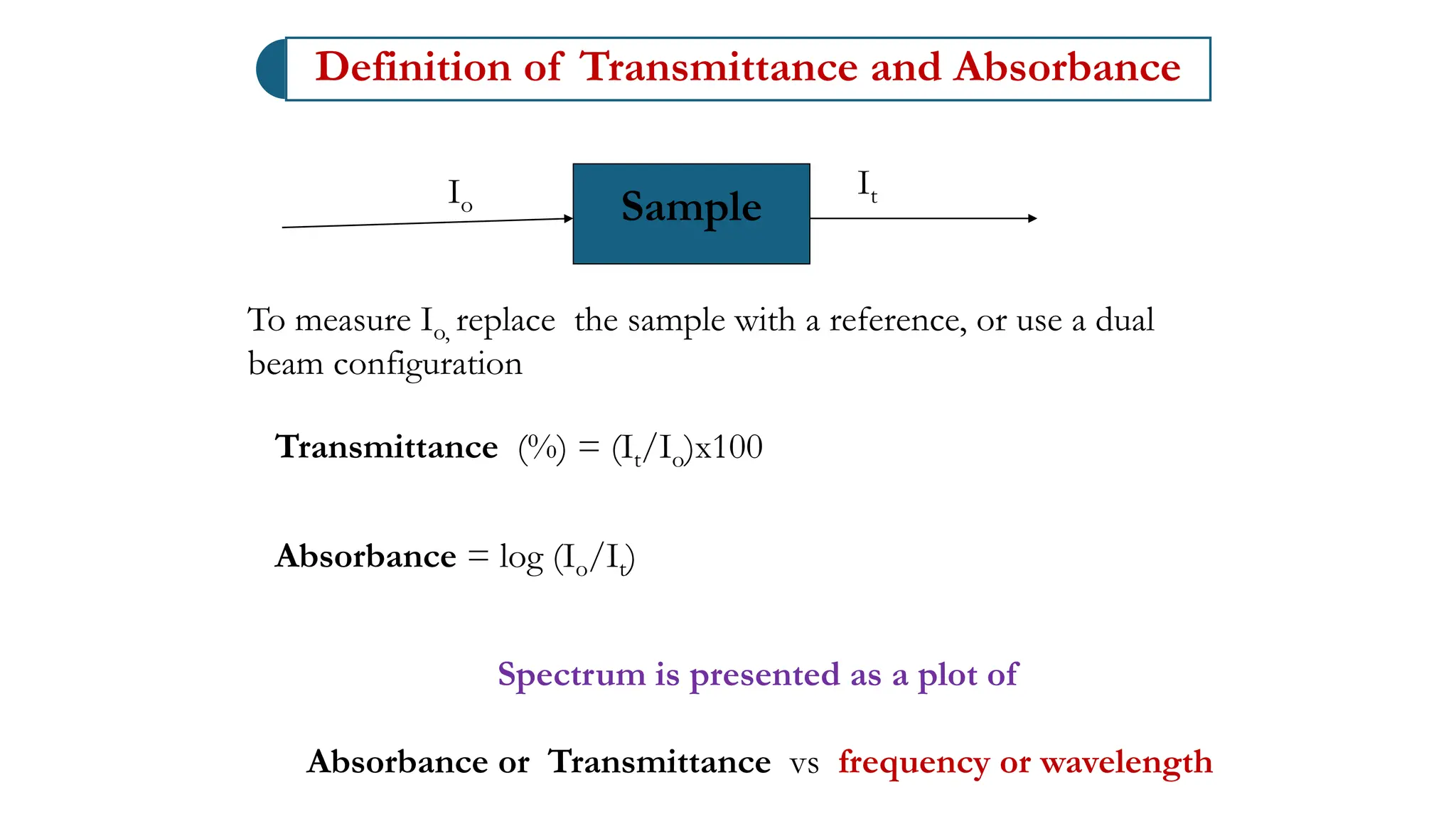
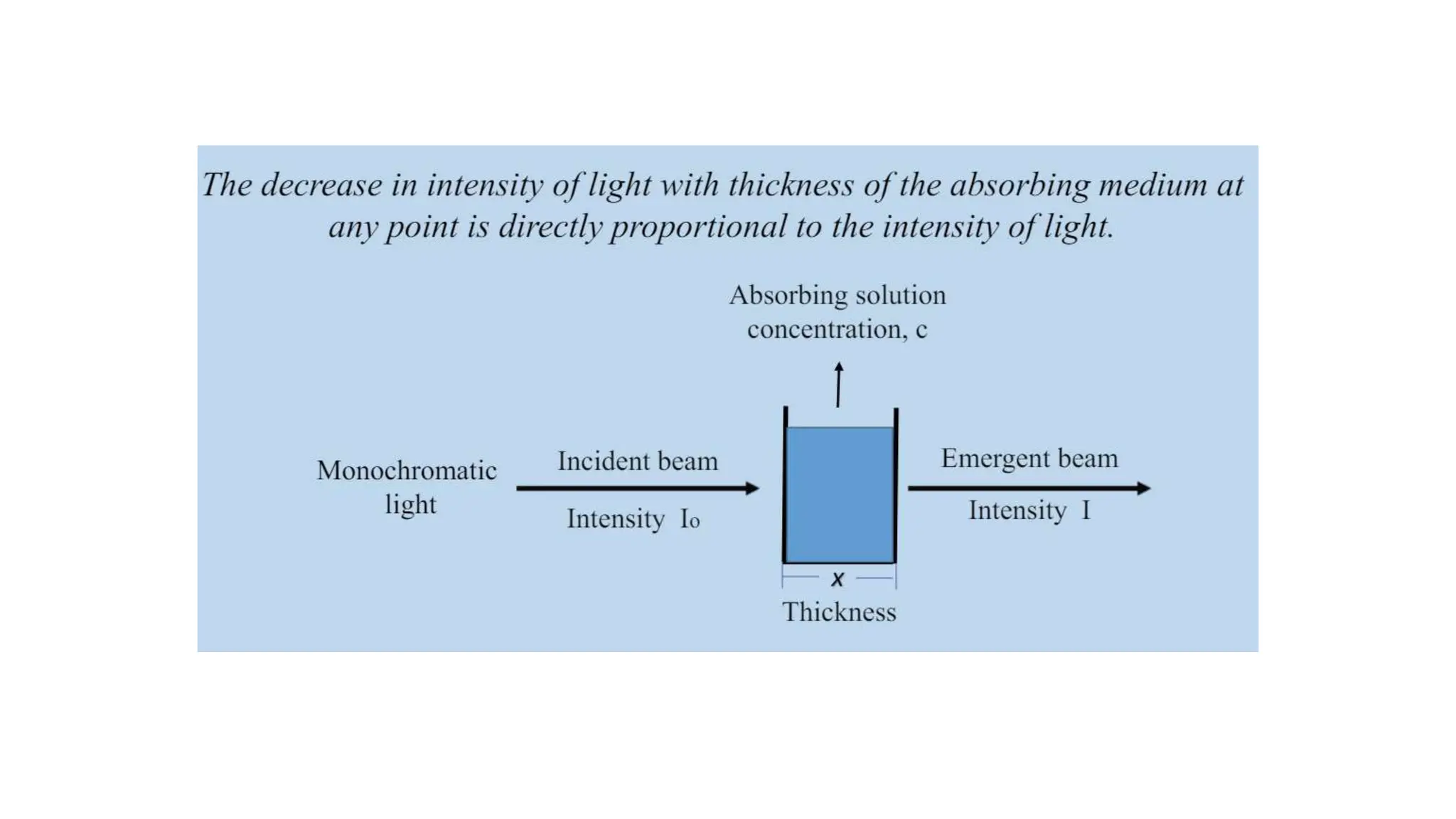
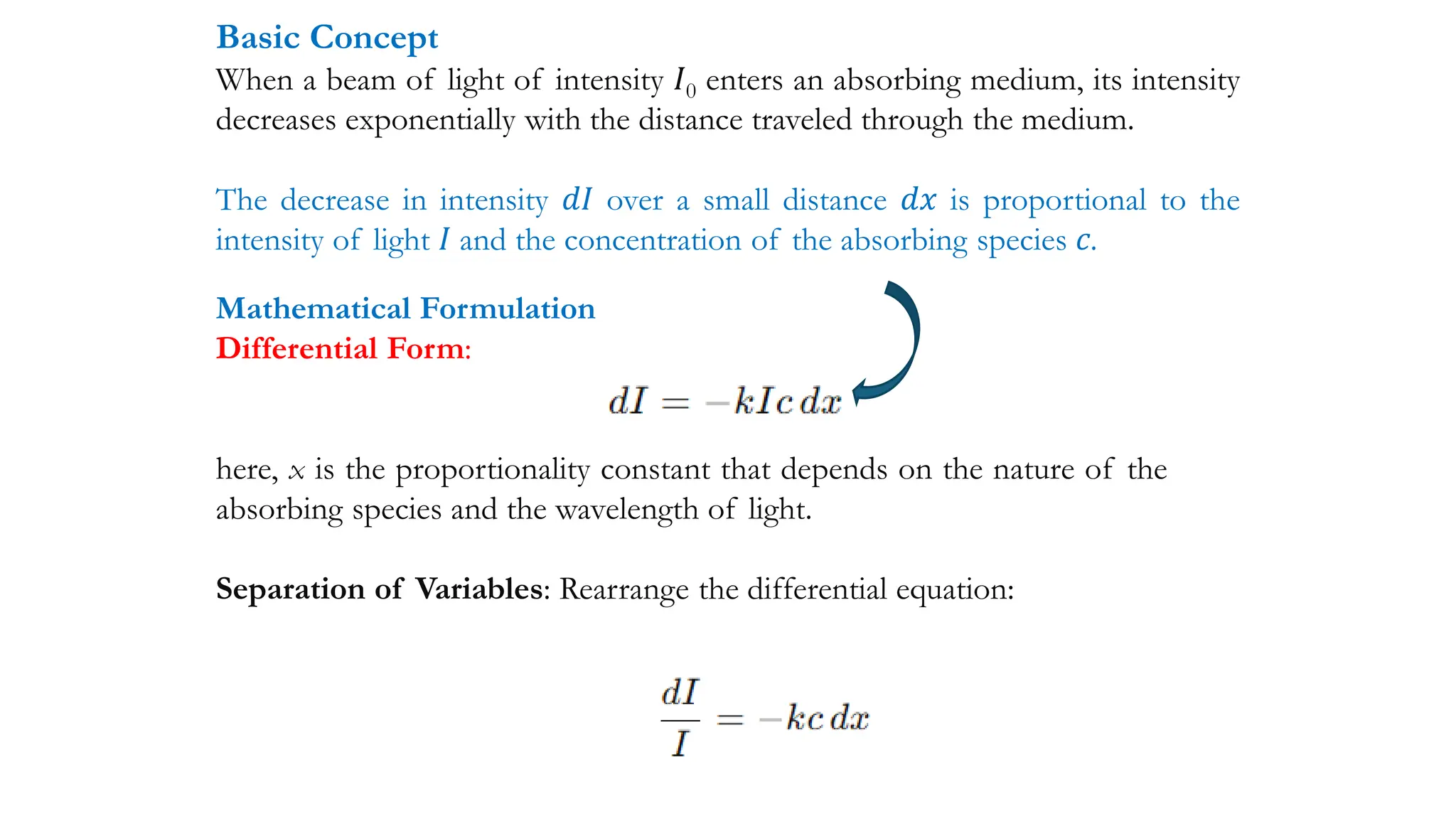
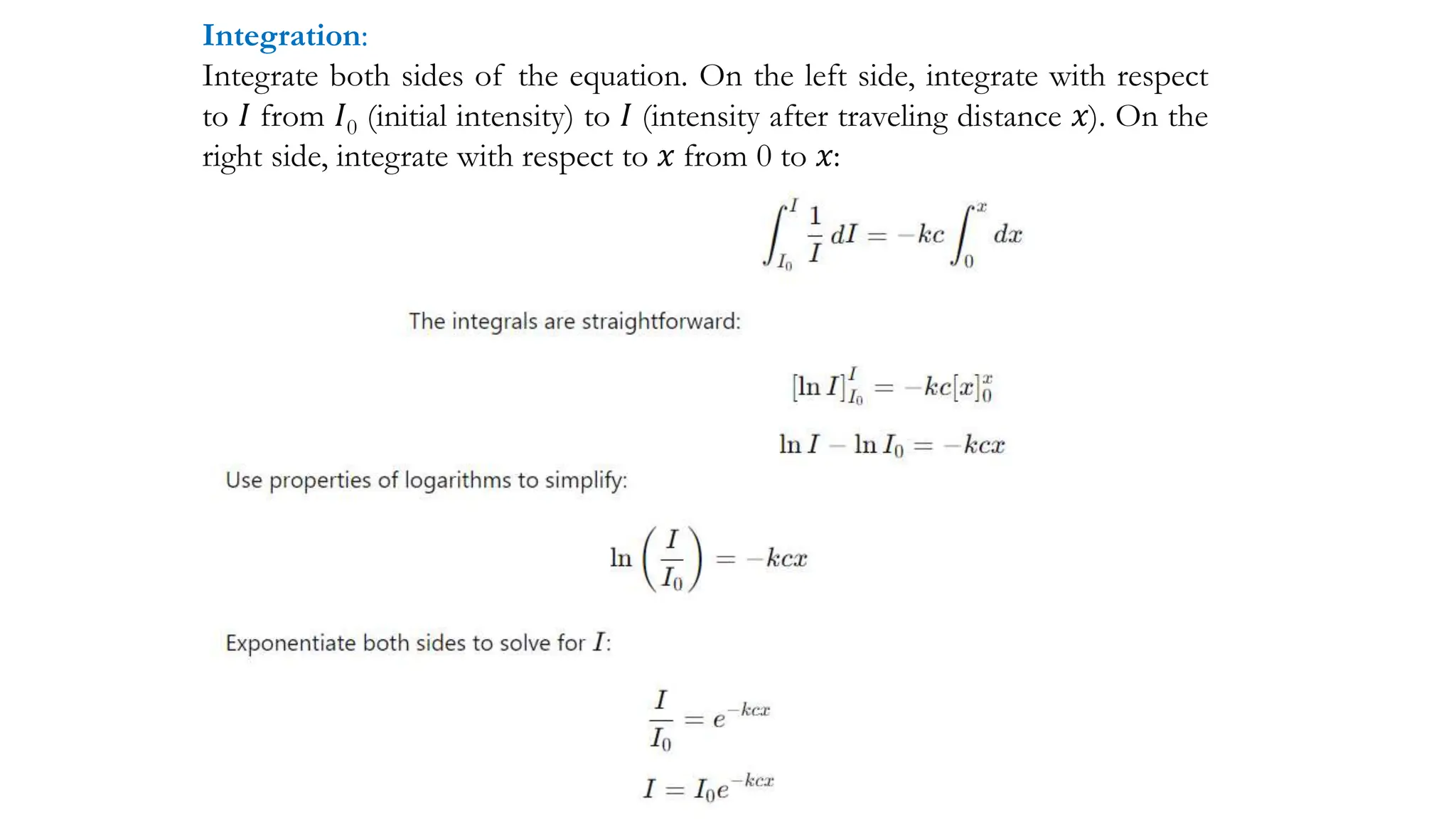
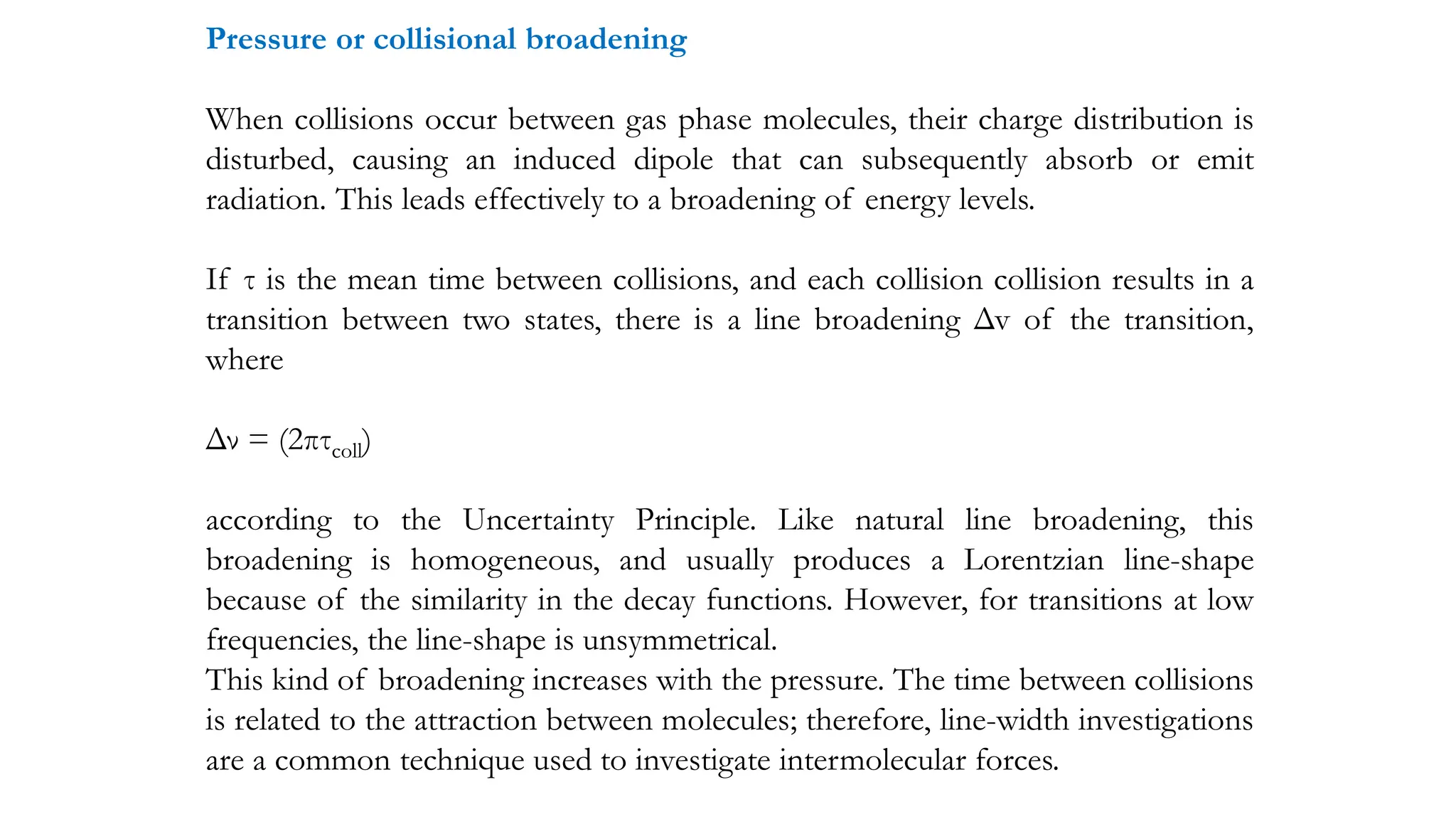
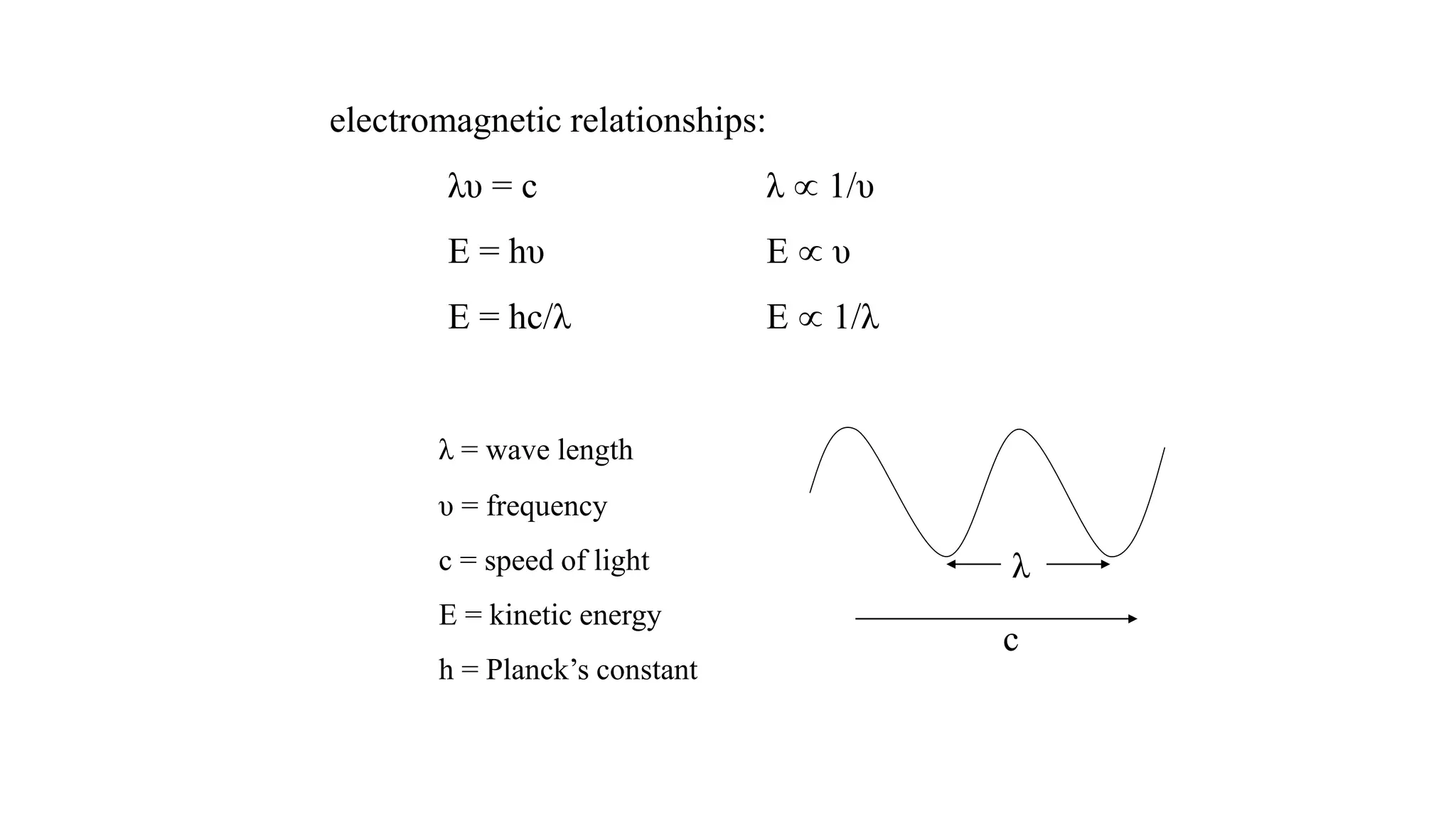
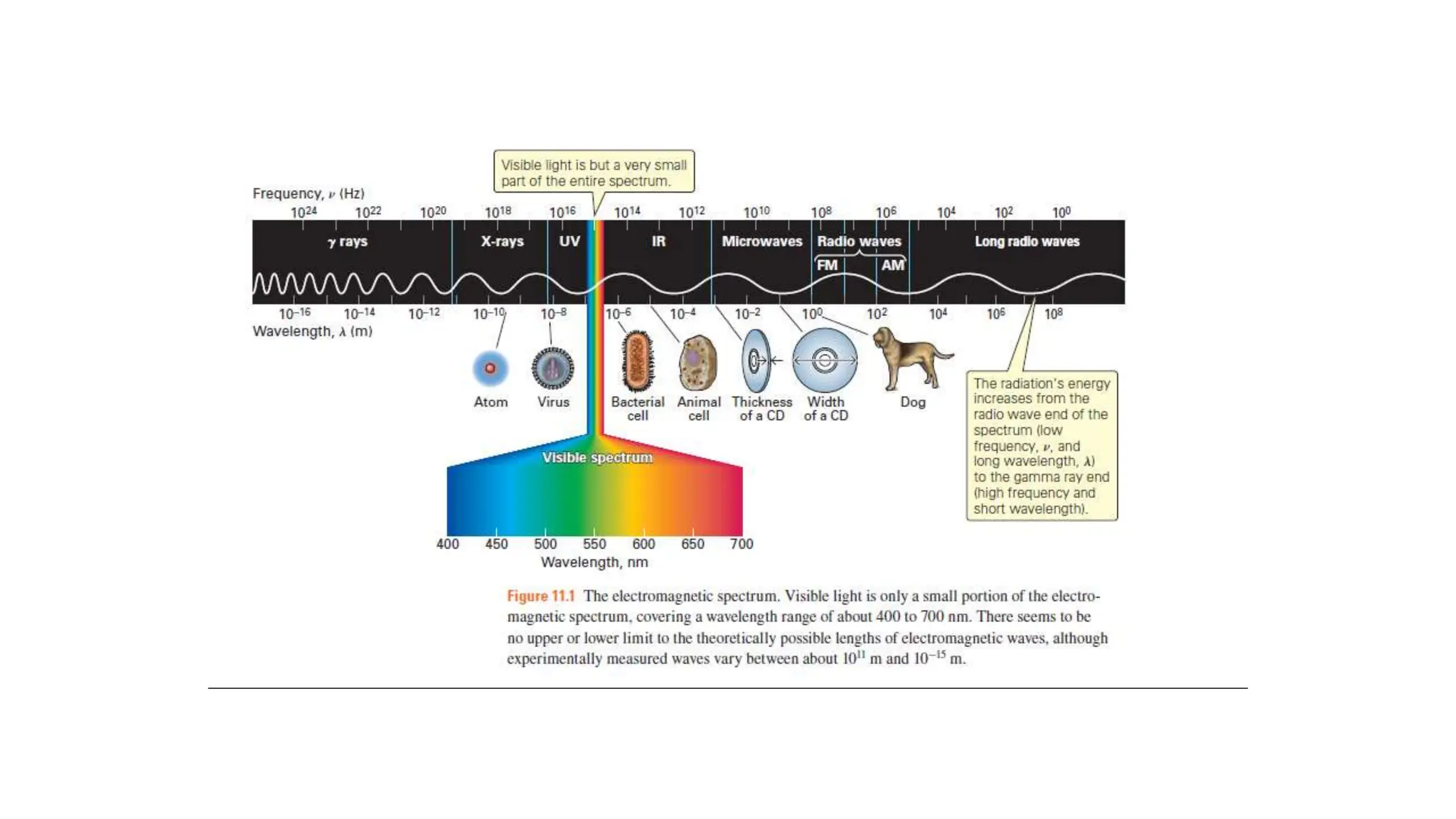
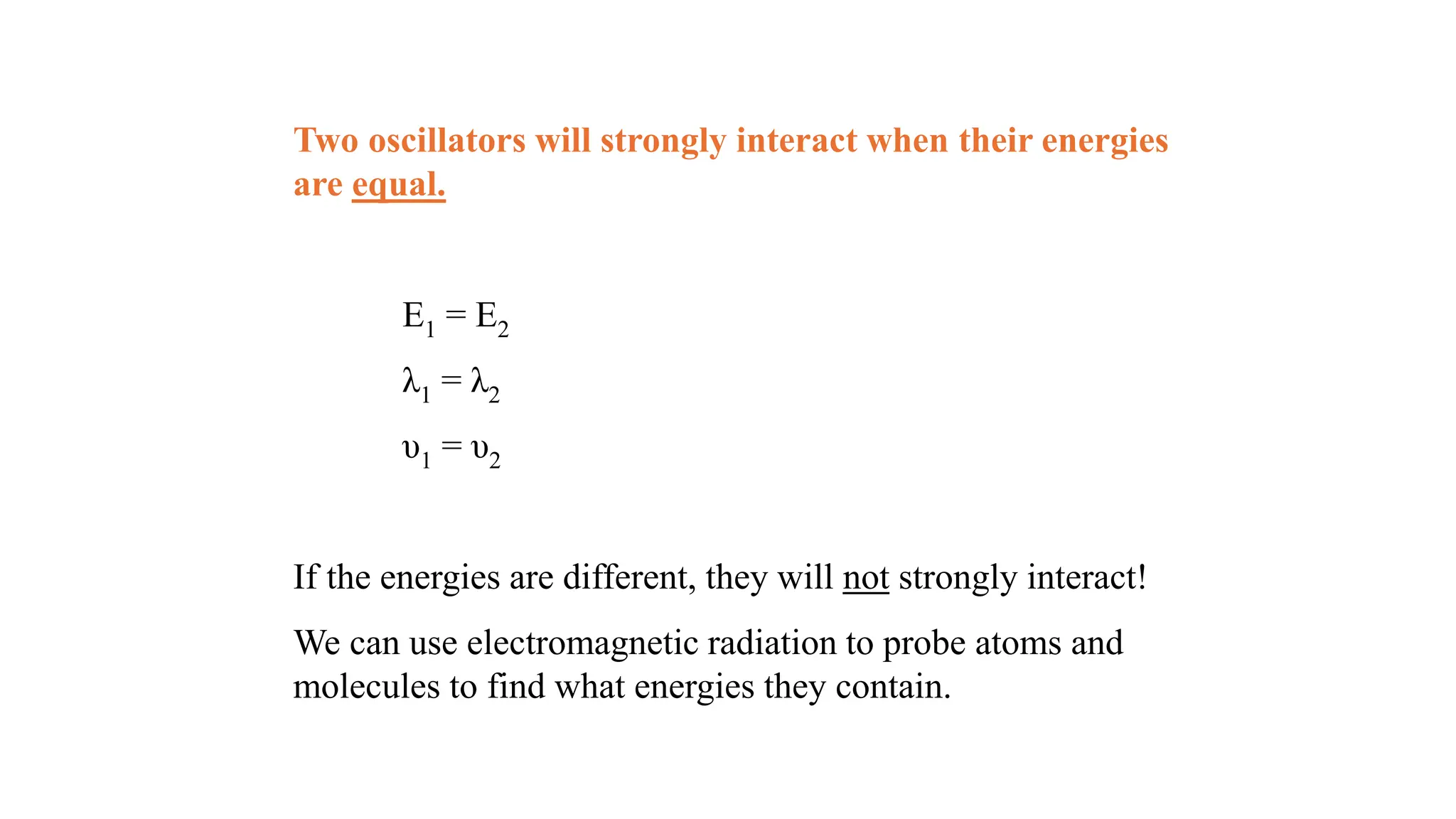
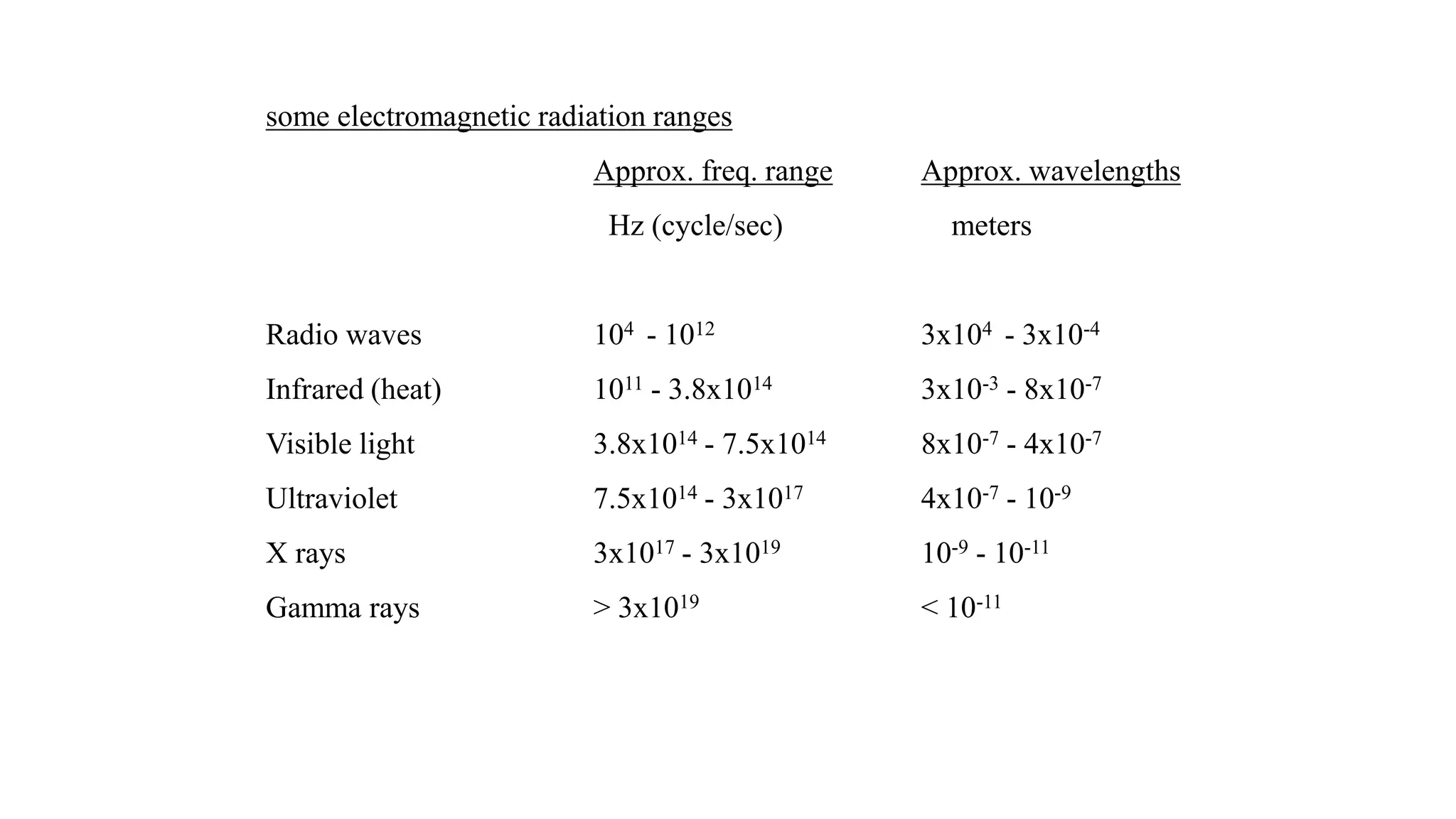
The document discusses electromagnetic radiation, detailing Maxwell's theory, the behavior of light, and key concepts such as wavelength, frequency, and the Beer-Lambert law, which relates light absorption to material properties. It explains how light can be polarized, monochromatic, or polychromatic, and outlines experimental methods for measuring light intensity and dispersion. Additionally, it addresses spectral line broadening and the relationship between molecular motion and spectroscopic techniques.