Embed presentation
Downloaded 126 times

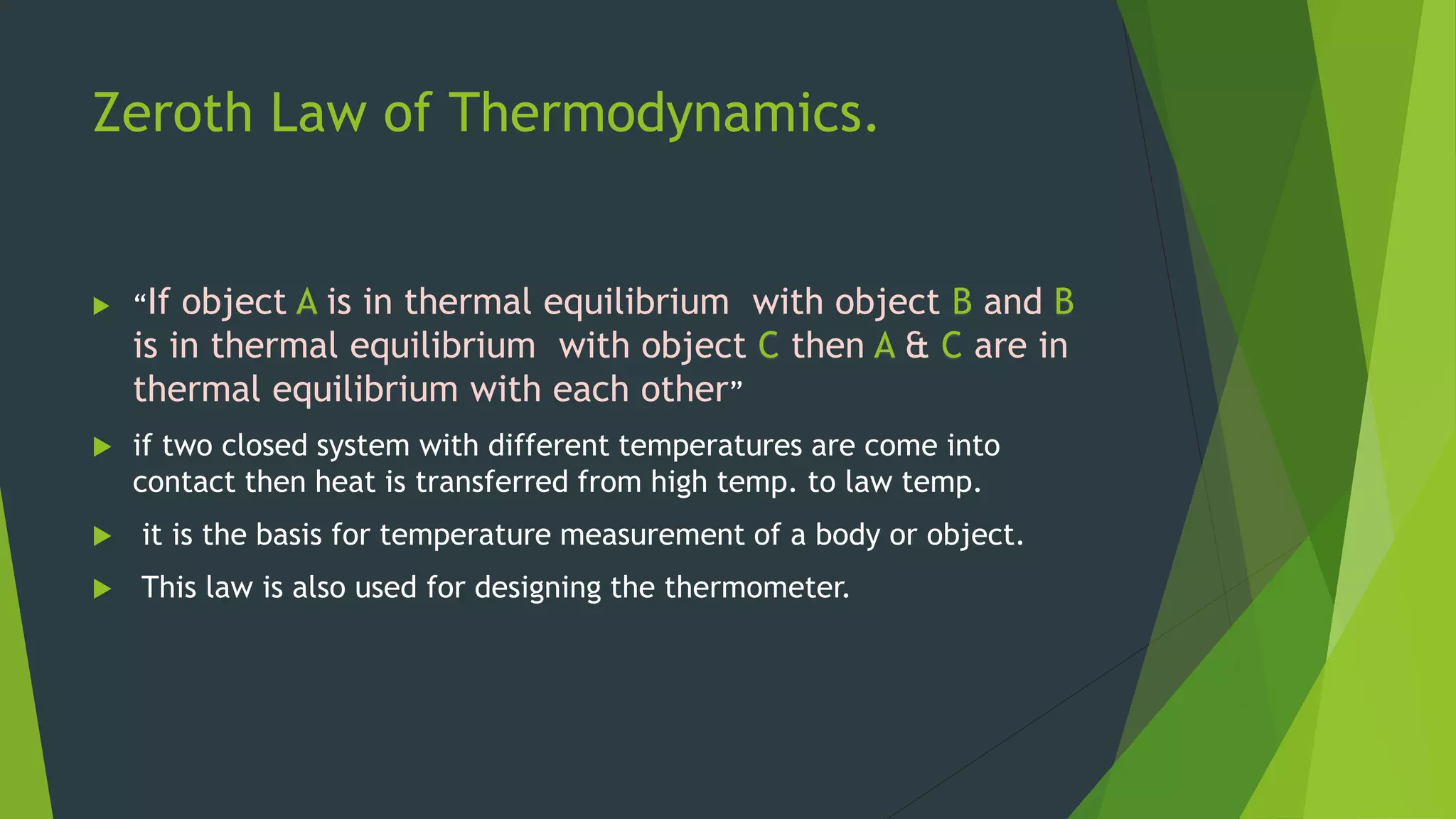






The document summarizes the four laws of thermodynamics: 1) The zeroth law establishes that thermal equilibrium is transitive. 2) The first law states that energy is conserved and relates heat, work, and changes in internal energy. 3) The second law says entropy always increases in spontaneous processes and Gibbs free energy always decreases. 4) The third law establishes that entropy reaches a minimum, zero, at absolute zero temperature.








