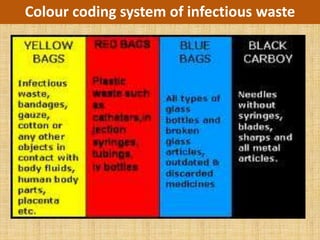
The document discusses good practices for working in a laboratory, including ensuring quality, ethics, waste management, and care of equipment. It emphasizes establishing standard operating procedures, training personnel, calibrating equipment, and documenting all studies to ensure reliability and reproducibility of results. Adhering to laboratory accreditation standards enhances competence and builds confidence in test results. Proper management of biomedical waste and ethical treatment of samples and patient information are also vital components of effective laboratory work.