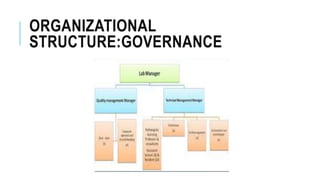
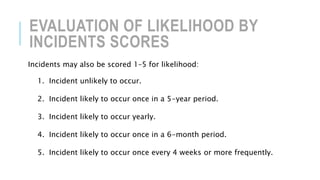
This document discusses various aspects of laboratory management and administration. It covers topics such as governance, operational units, organizational structure, risk management, quality management, and accreditation standards. Specifically, it provides details on identifying and assessing risks, developing quality management systems, and the importance of accreditation to ISO standards for medical laboratories. The overall aim is to ensure laboratories have effective systems in place to minimize risks, maintain quality of services, and meet regulatory requirements.