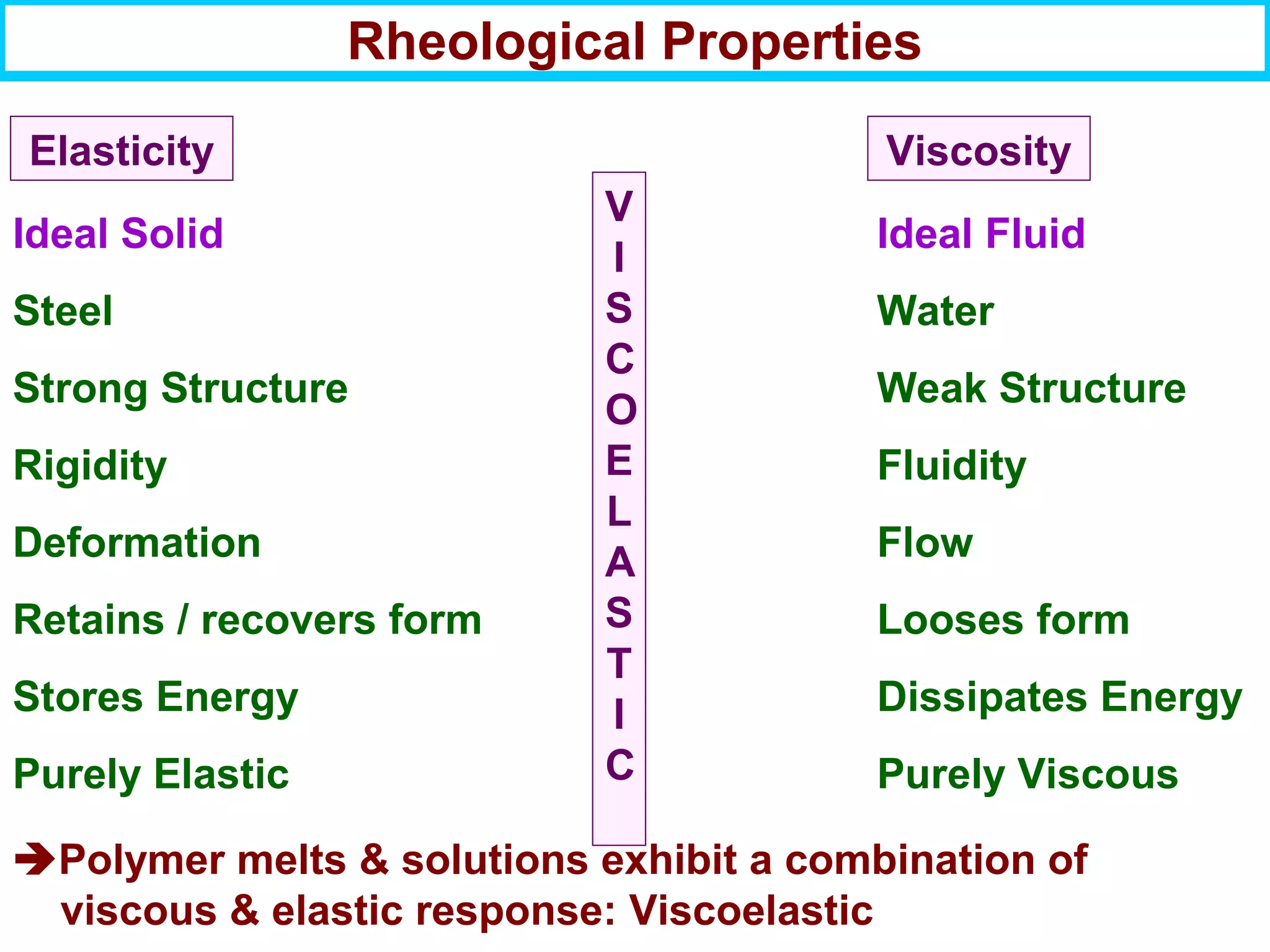
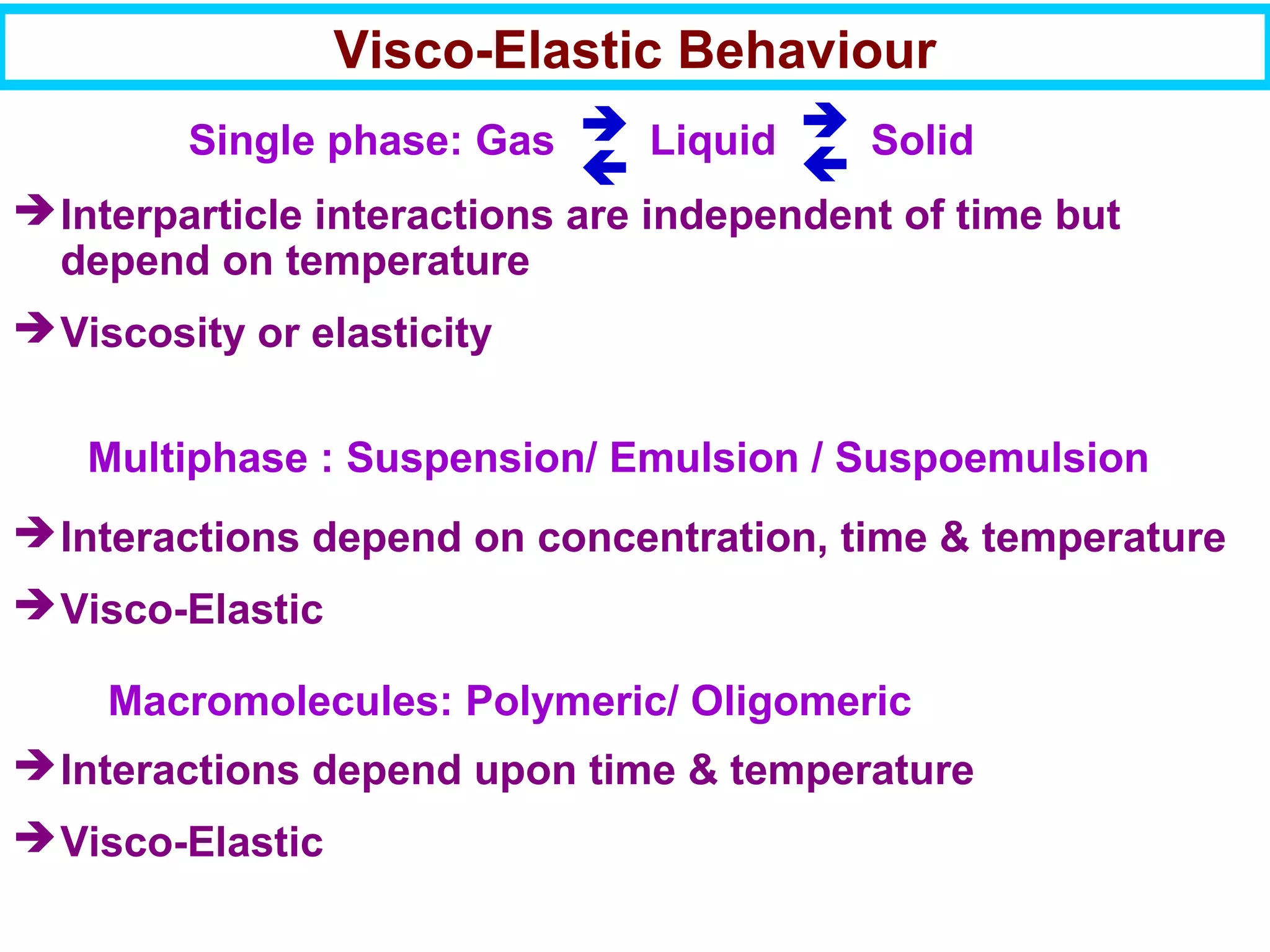
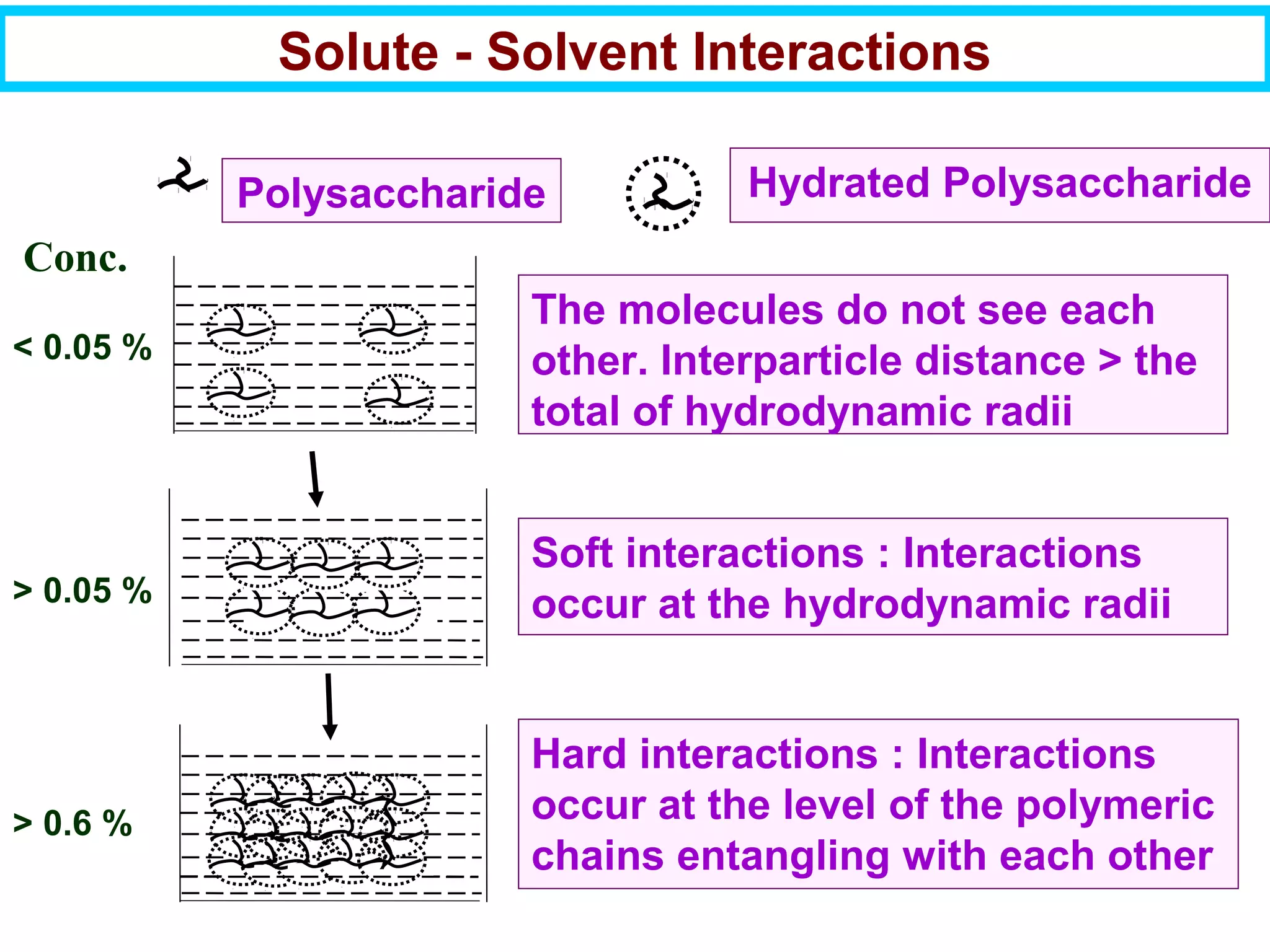
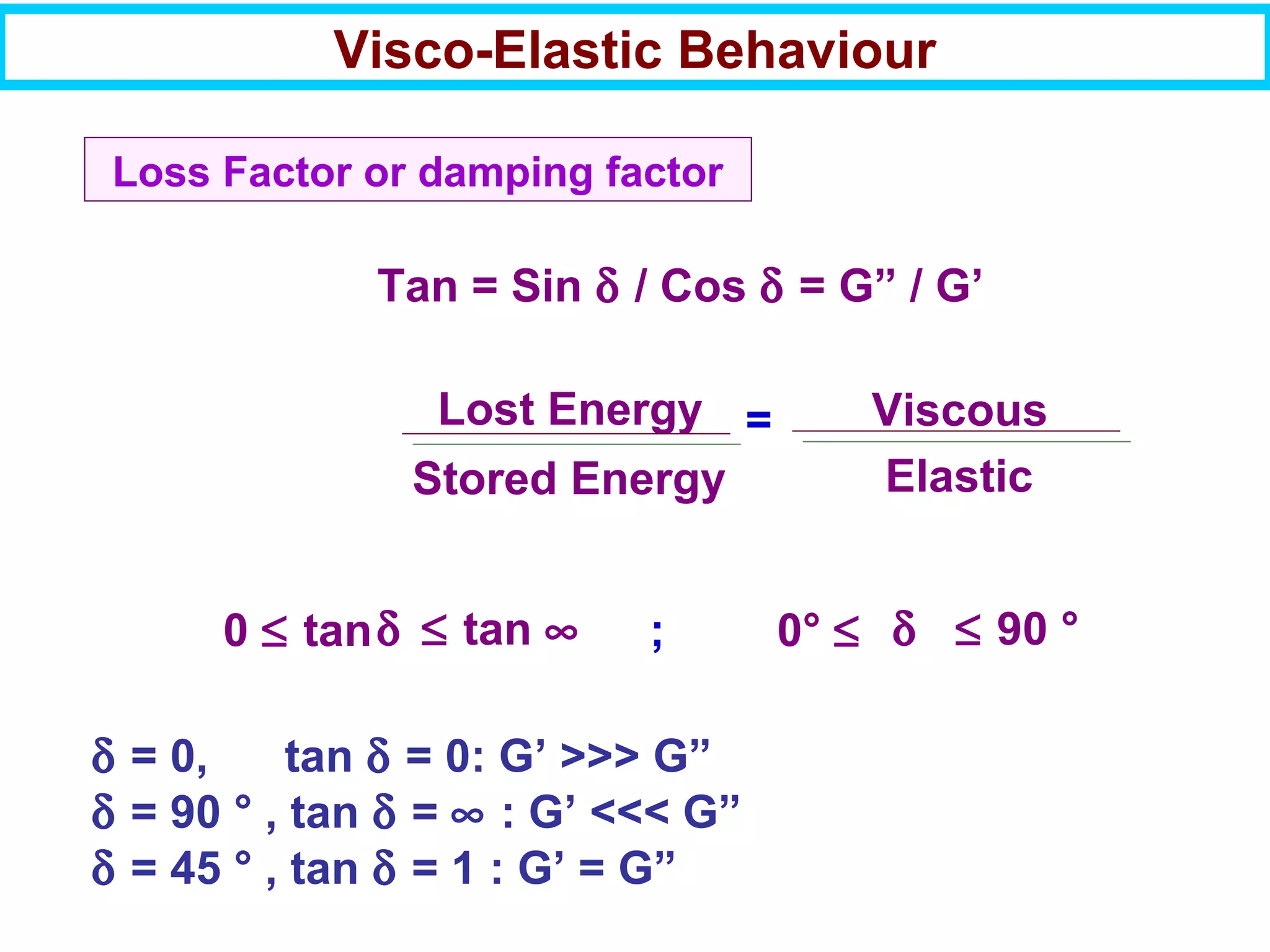
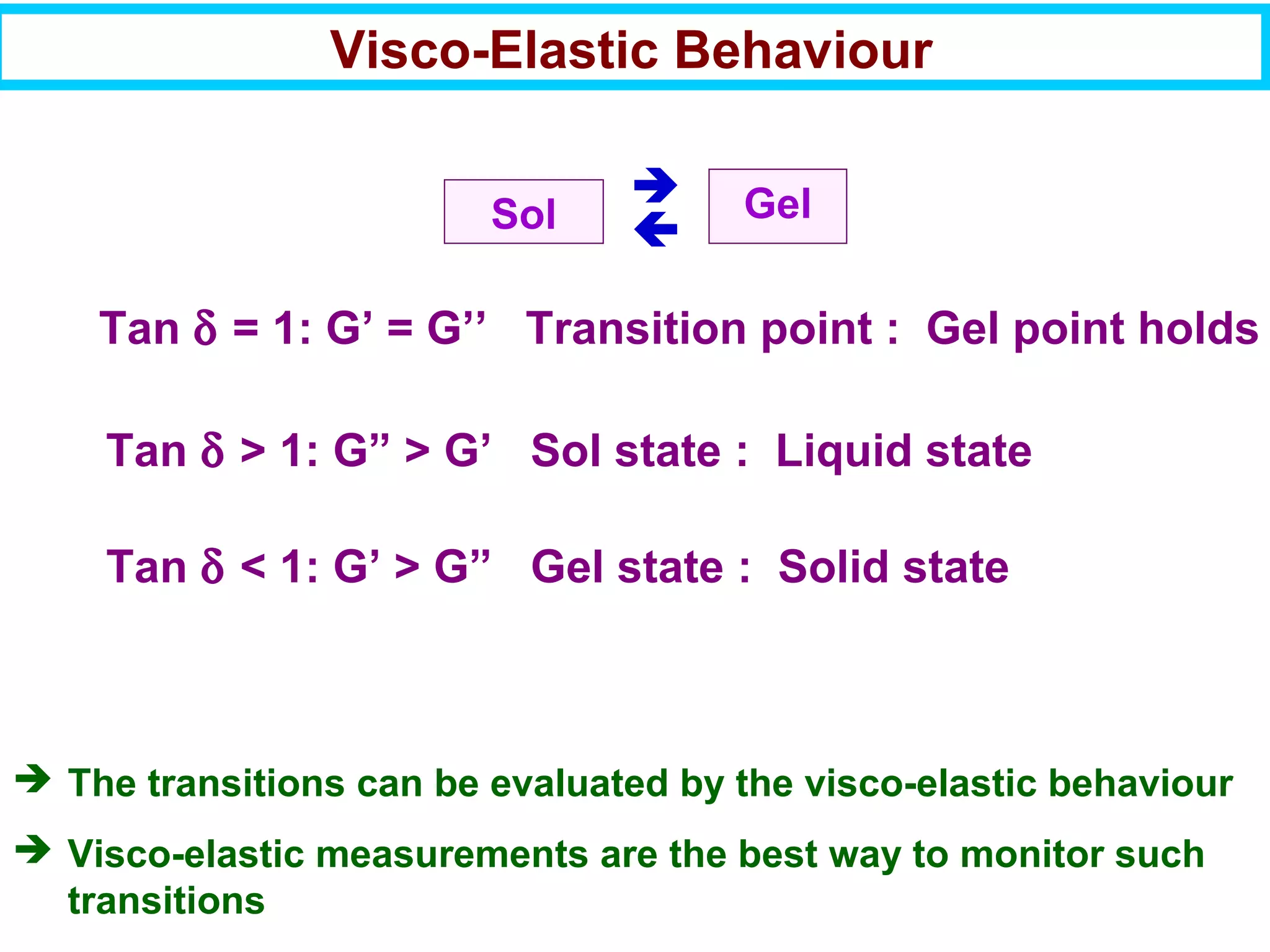
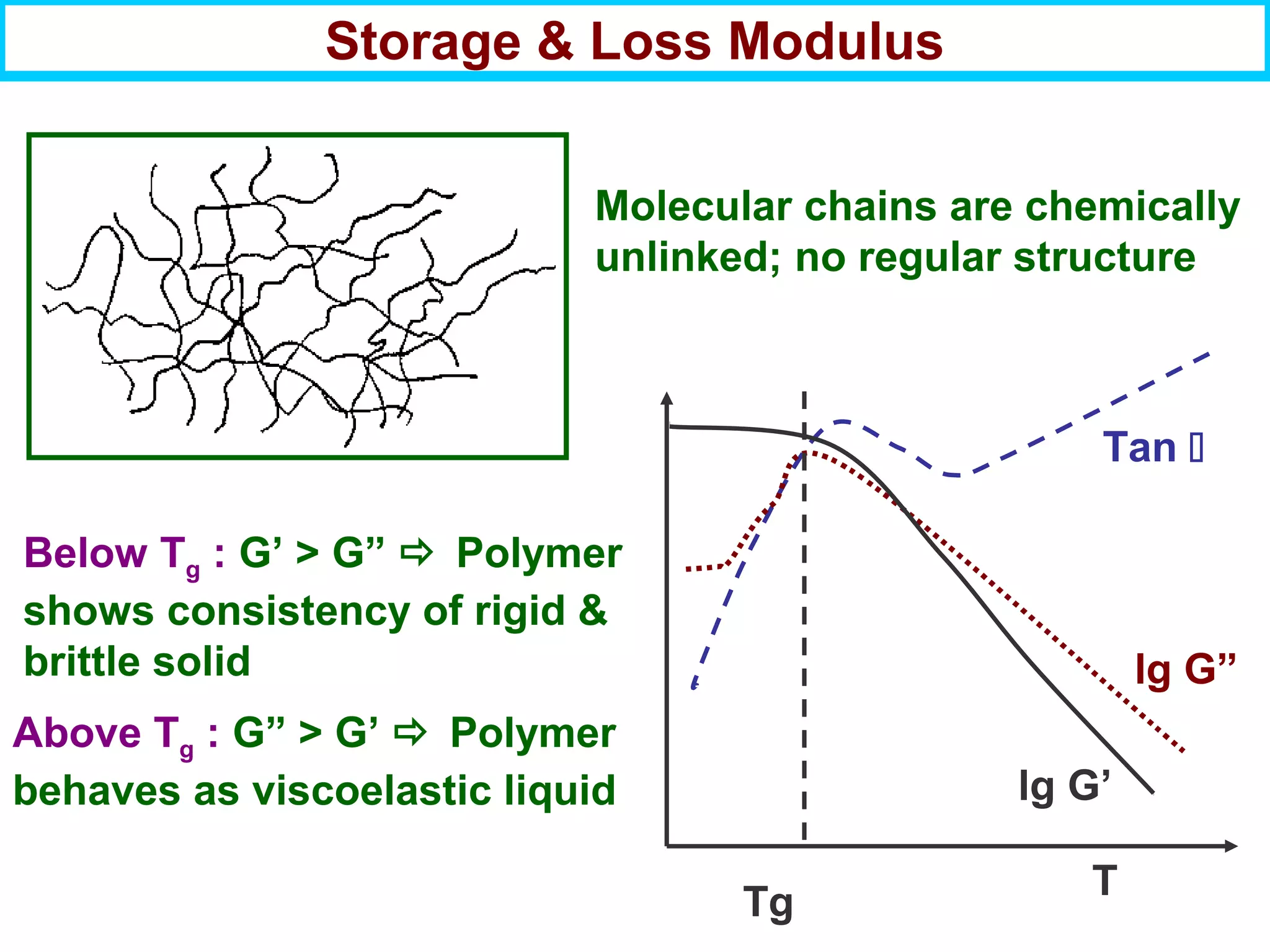
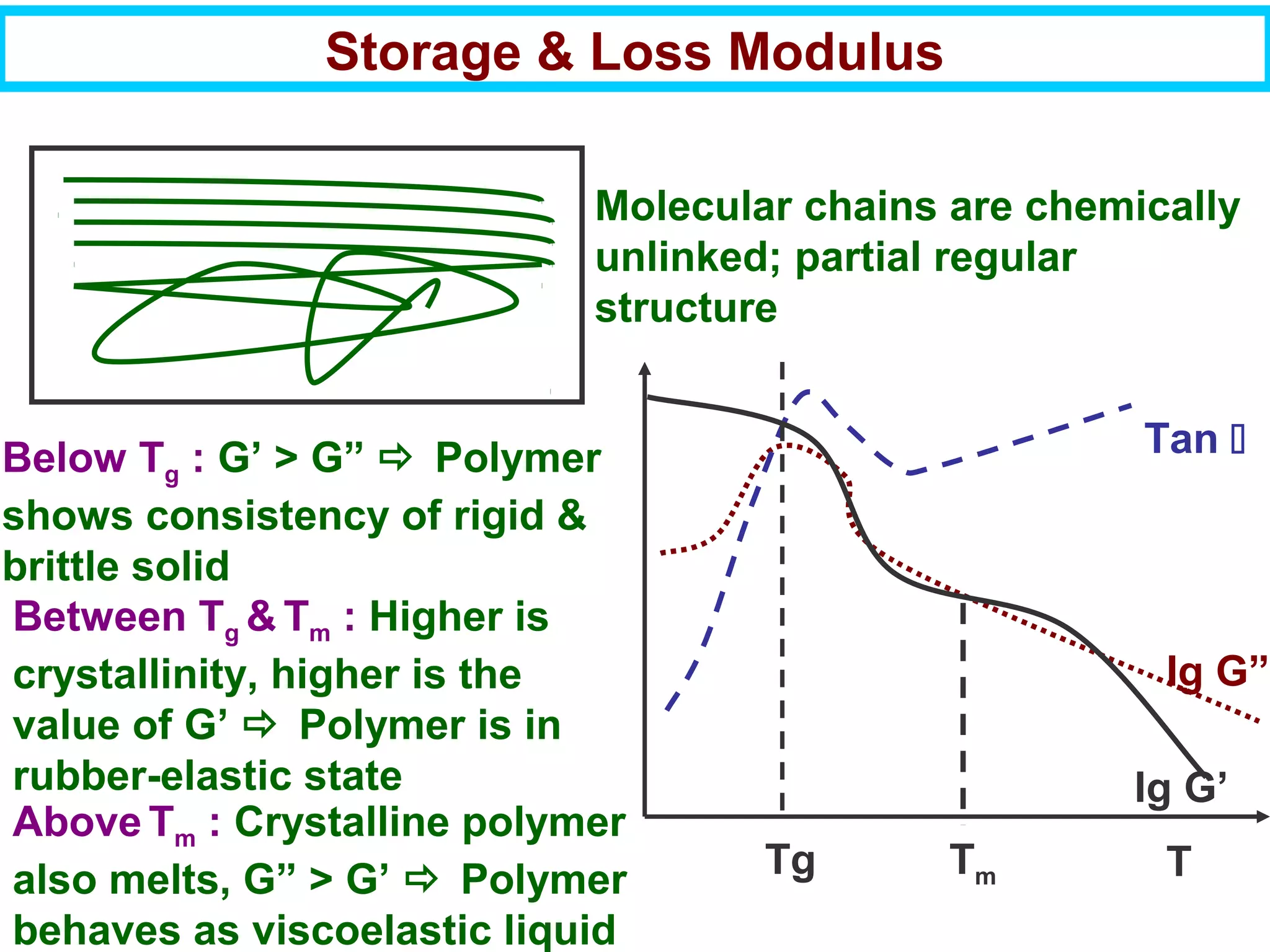
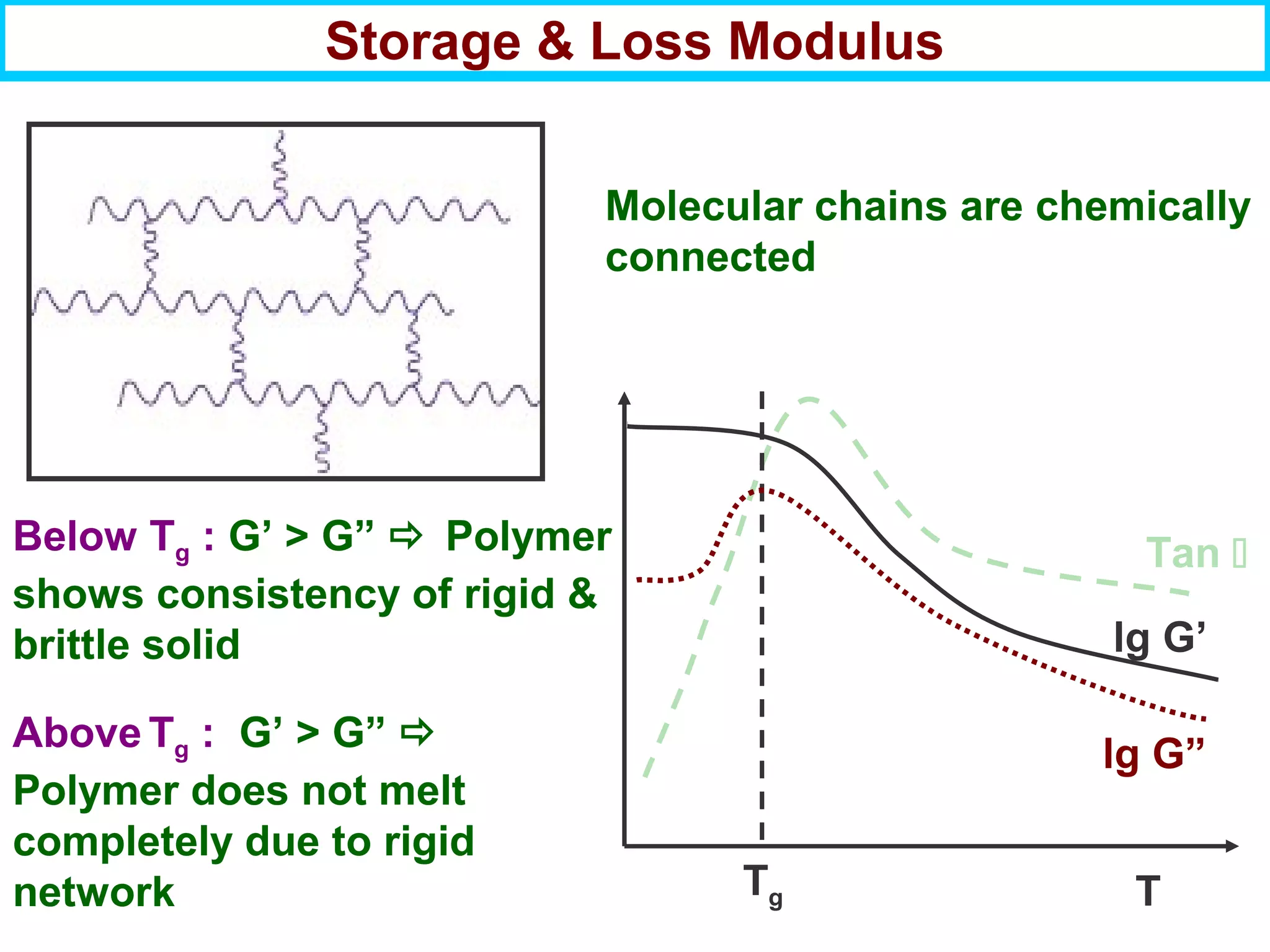
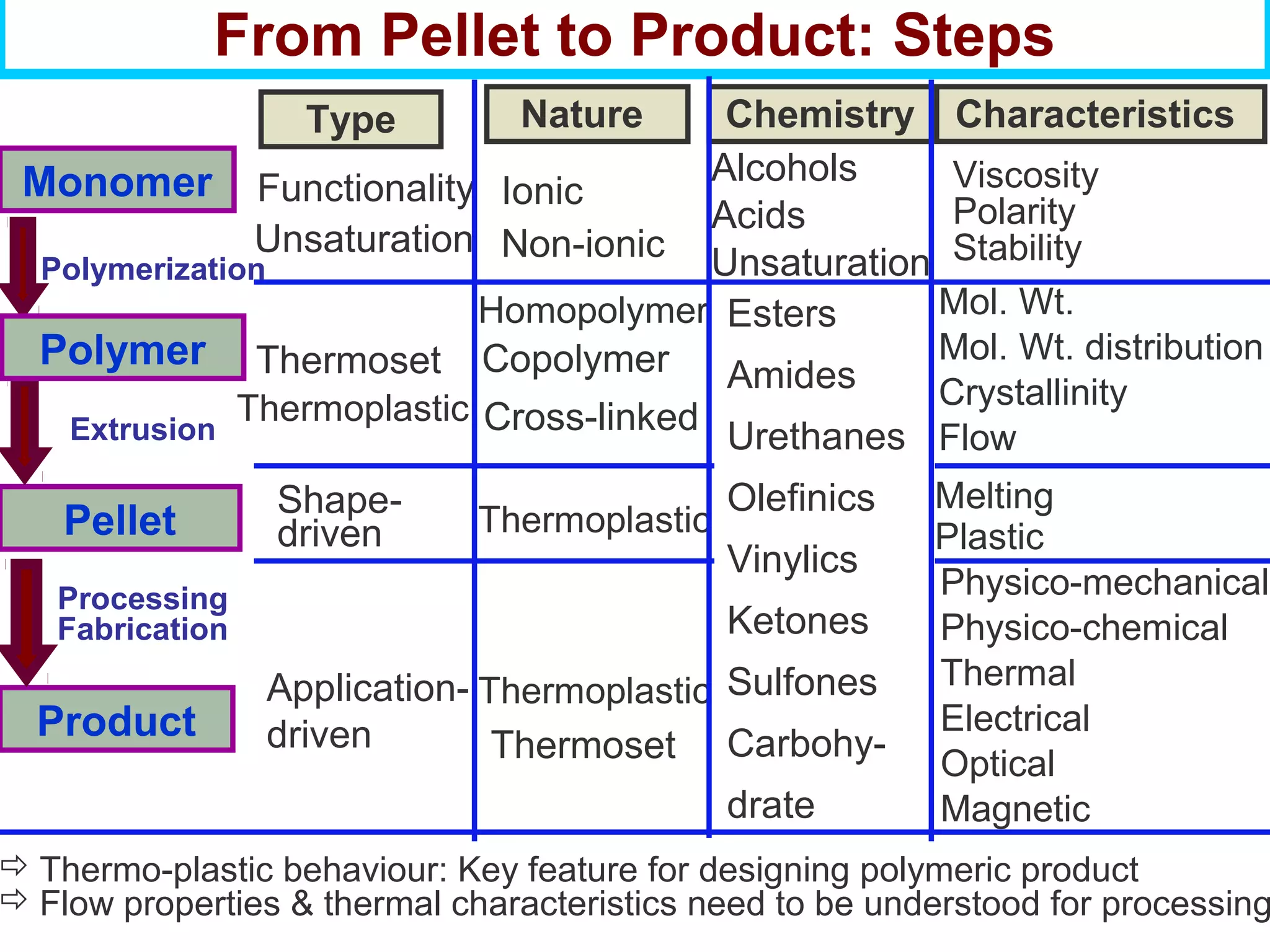
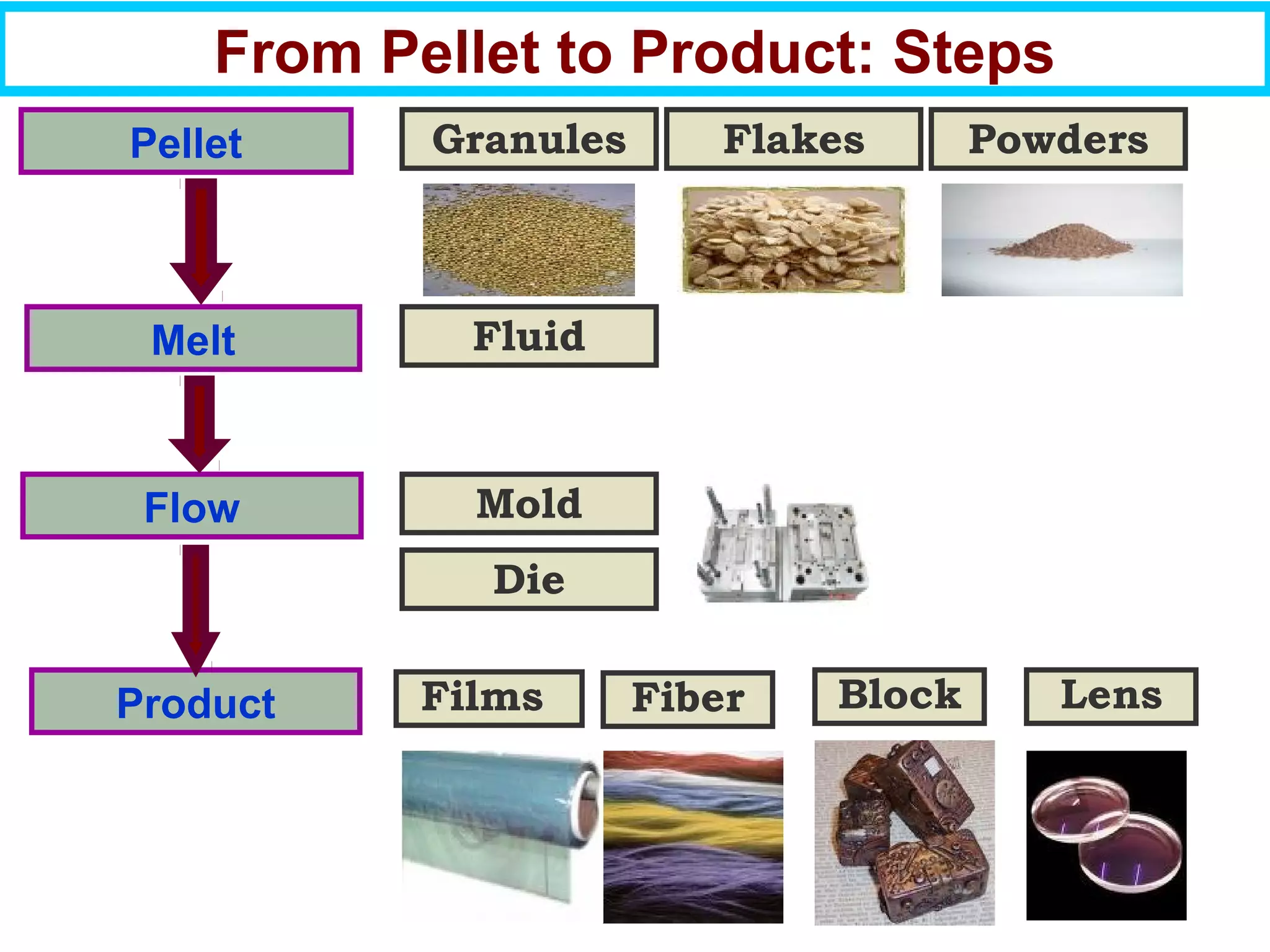
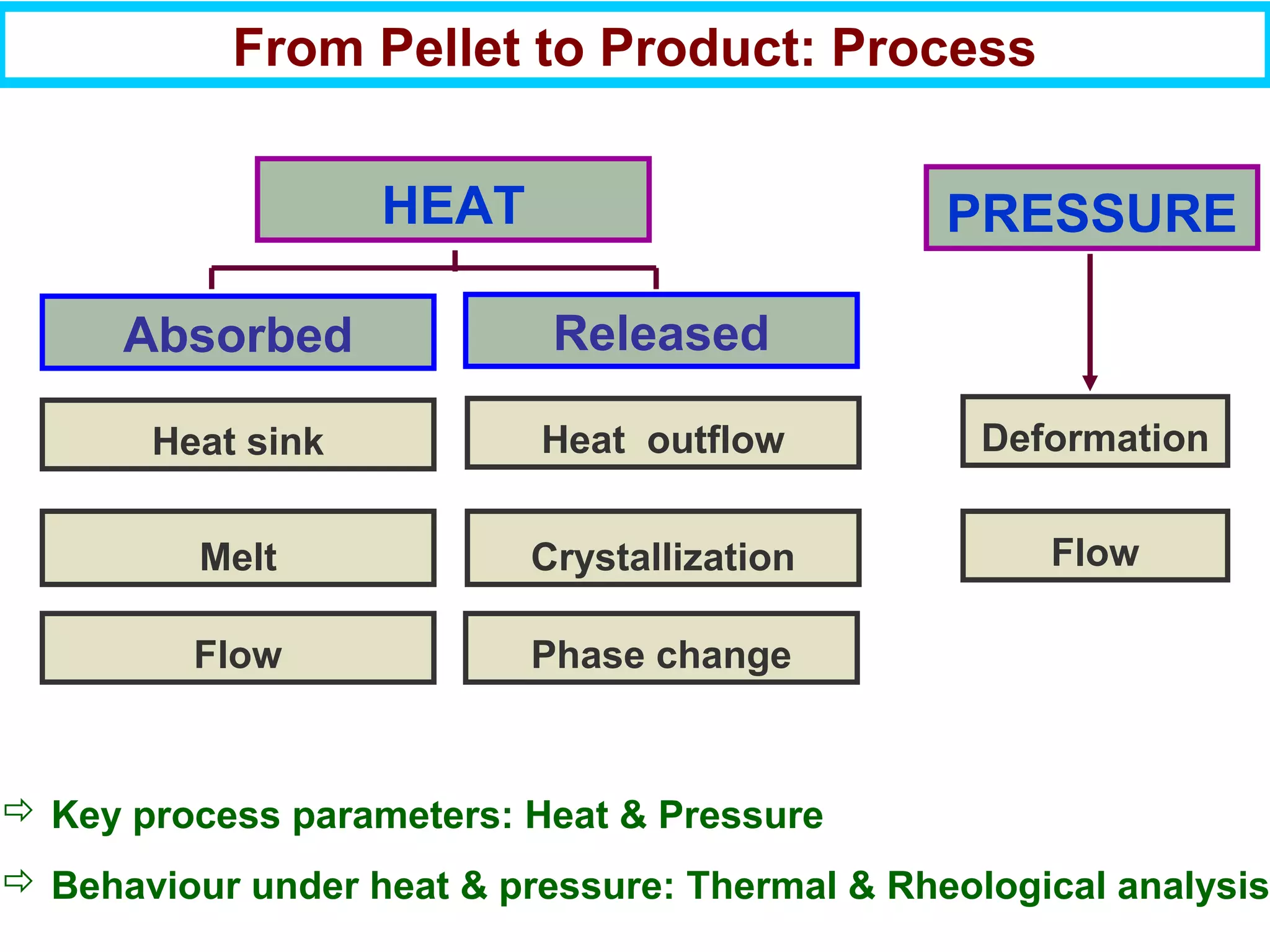
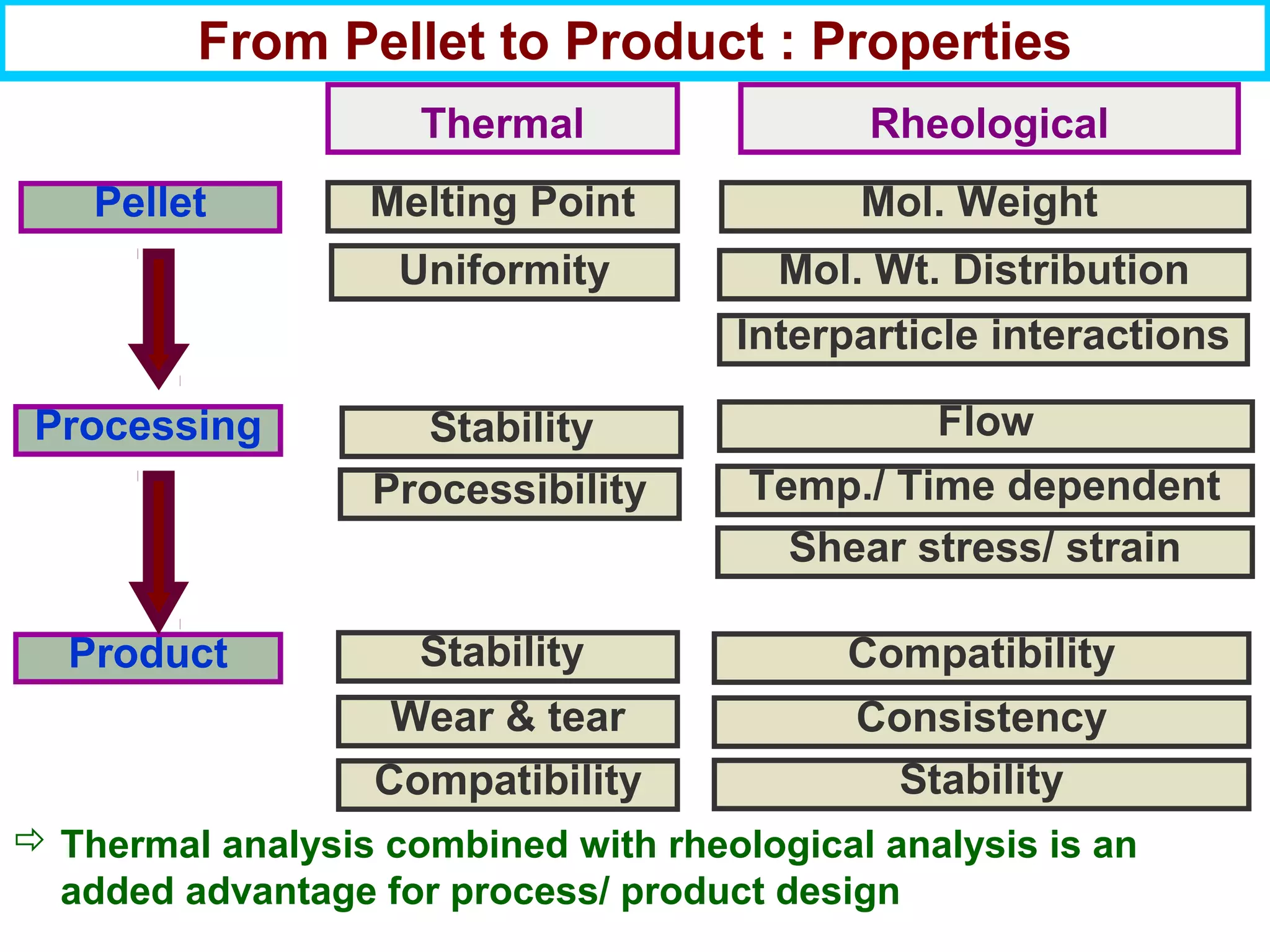
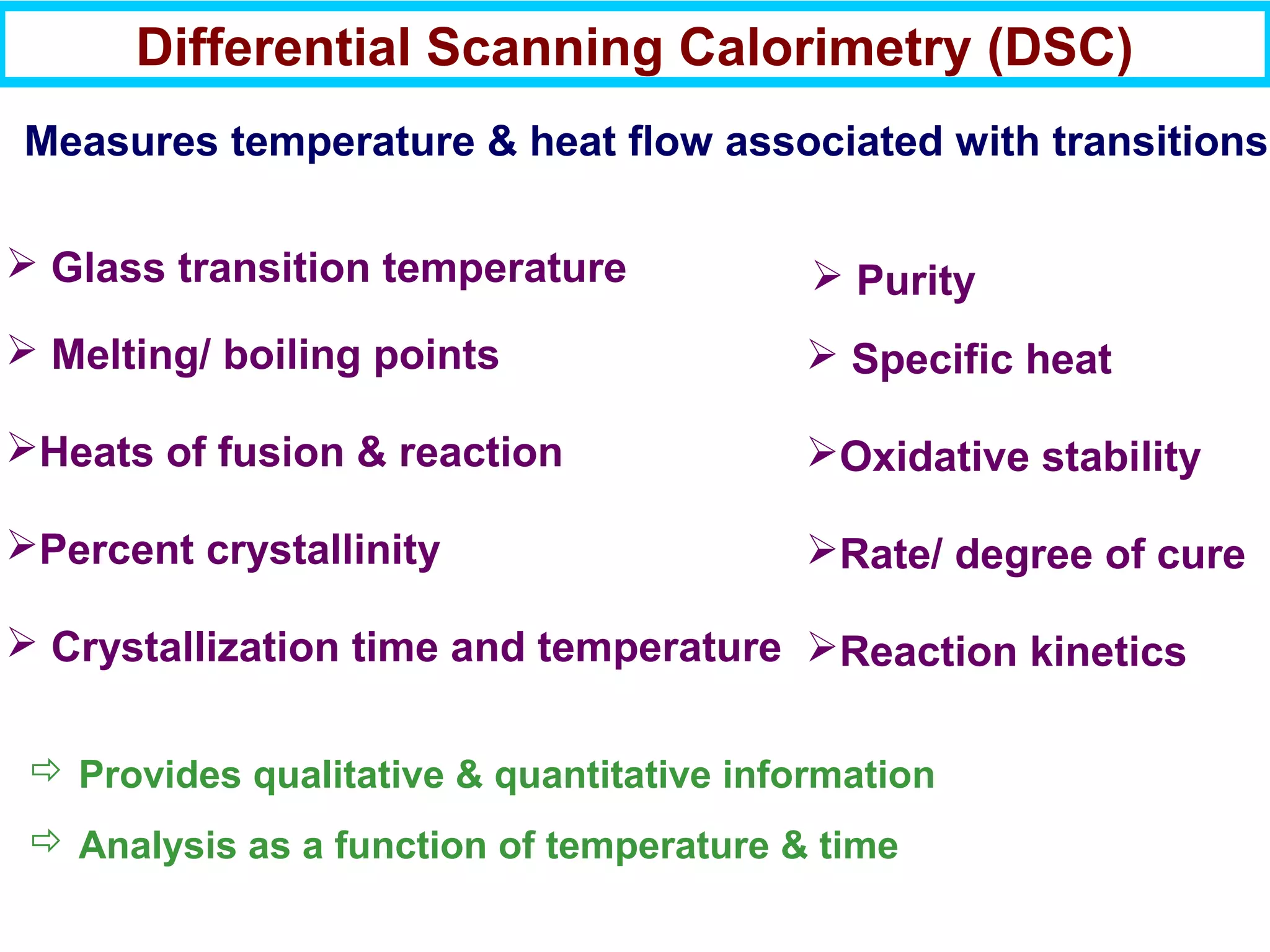
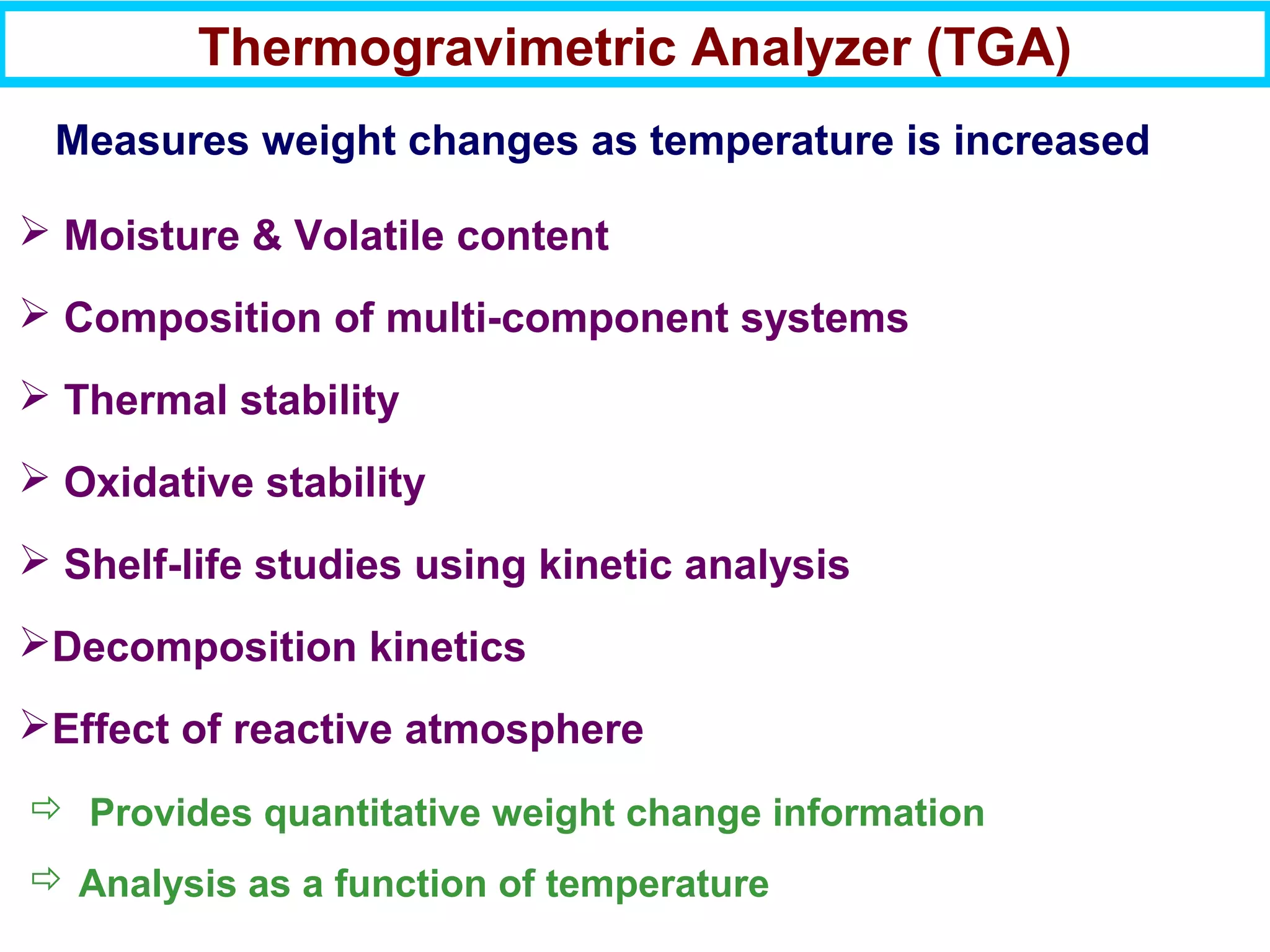
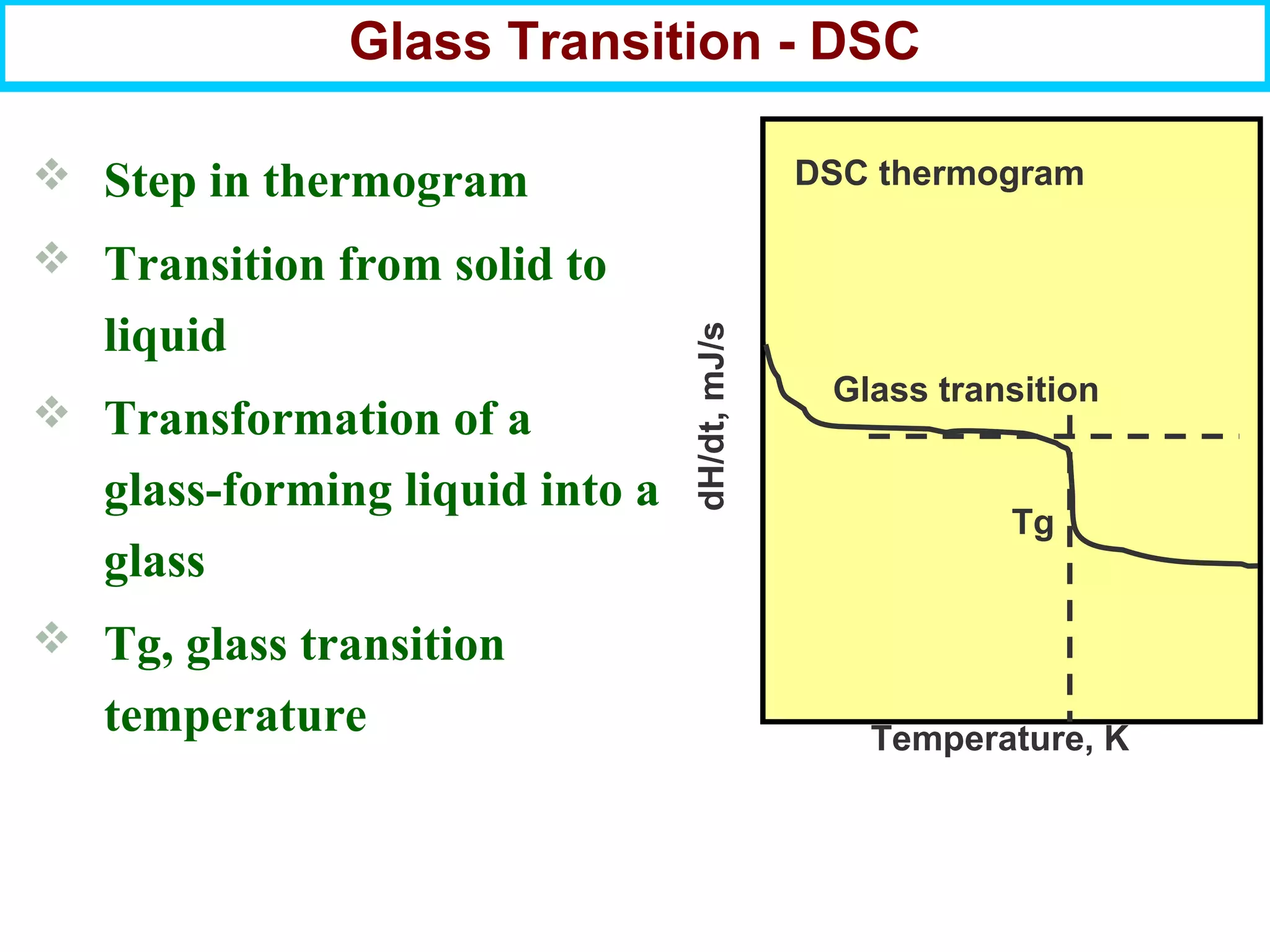
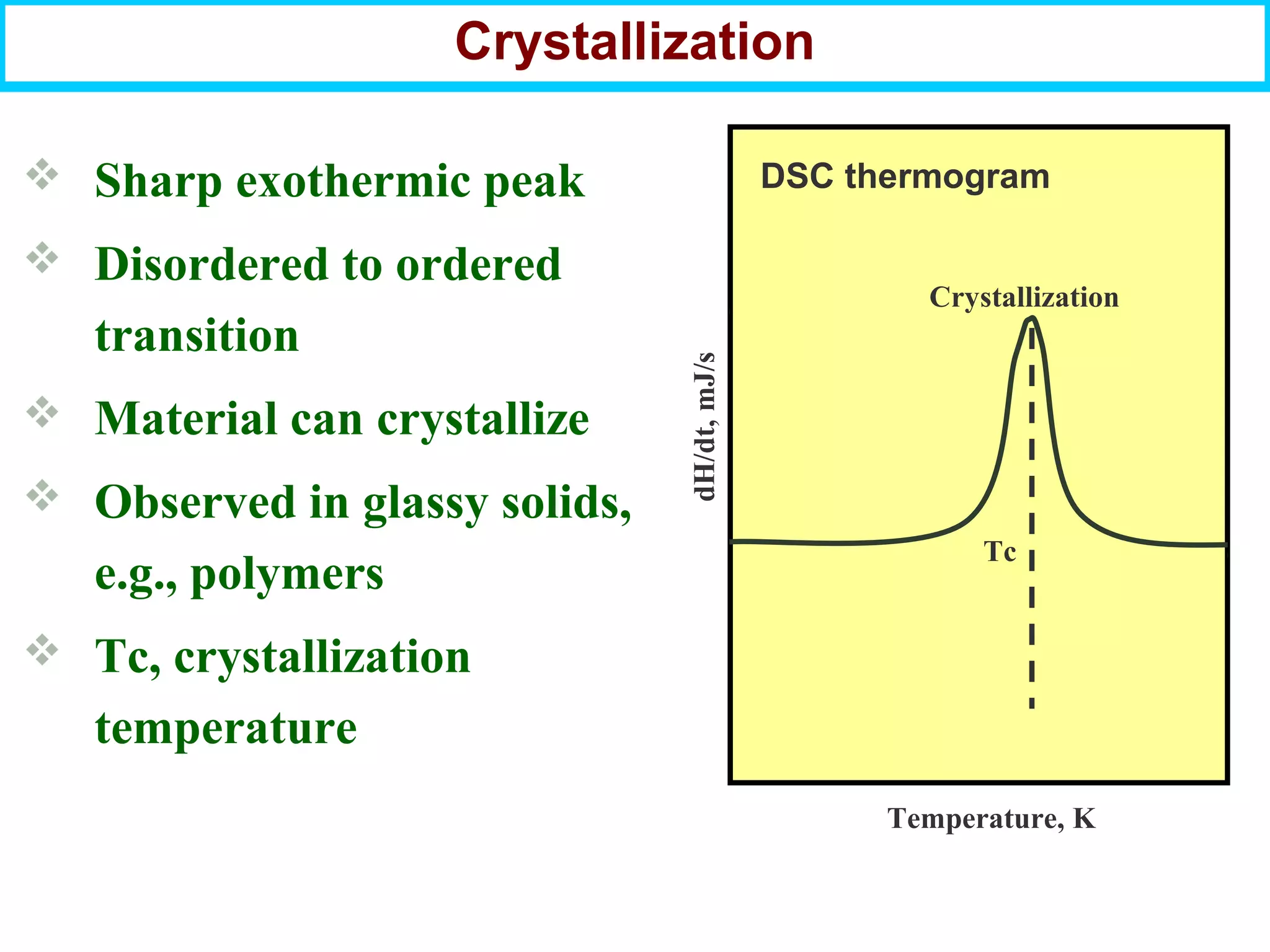
This document discusses the key steps and properties involved in transforming polymer pellets into finished products. It outlines the process from pellet to product, including polymerization, extrusion, processing, fabrication, and the resulting properties. The document emphasizes the importance of thermal and rheological analysis for understanding the flow properties and thermal characteristics needed for processing. It describes techniques like differential scanning calorimetry and thermogravimetric analysis for thermal analysis, and defines concepts like viscosity, viscoelastic behavior, and storage and loss modulus for rheological analysis. The document argues that thermal analysis combined with rheological analysis provides an advantage for process and product design.


![Newtonian
Example :Oil,Water
Shear rate γ•
[1/ S]
Shearstressτ[Pa]
Viscosity is independent of shear rate
Shear rate ∝ shear stress](https://image.slidesharecdn.com/keynote090310-140626085038-phpapp01/75/Keynote090310-14-2048.jpg)
![Non-Newtonian: Dilatant
Shear-thickening or Dilatant
Shear rate[1/S]
Shearstressτ[Pa]
Viscosity with in shear rate
Example: ceramic suspension, plastisol pastes, starch
dispersions](https://image.slidesharecdn.com/keynote090310-140626085038-phpapp01/75/Keynote090310-15-2048.jpg)
![Non-Newtonian: Pseudoplastic
Shear rate[1/ γ•
S]
Shear thinning: Example: Polymer melts, paints, glues.
Viscosity as shear rate
Material shows plasticity only after yield point
Above yield point Plasticity,below yield point elasticity
Shearstressτ[Pa]](https://image.slidesharecdn.com/keynote090310-140626085038-phpapp01/75/Keynote090310-16-2048.jpg)
![THIXOTROPY & RHEOPEXY
Time(t)
For Thixotropic
materials, viscosity
as time , at a constant
shear rate.
Example: Paraffin
oil,Pastes,cream,gels
For Rheopectic fluid ,
viscosity as time
at a constant shear
rate
Thixotropic
Rheopectic
Shearstressτ[Pa]](https://image.slidesharecdn.com/keynote090310-140626085038-phpapp01/75/Keynote090310-17-2048.jpg)