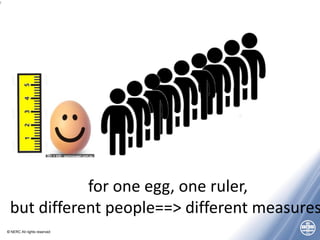
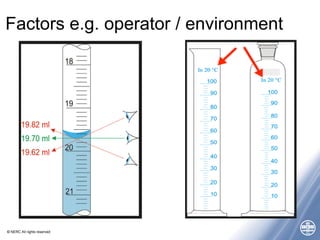
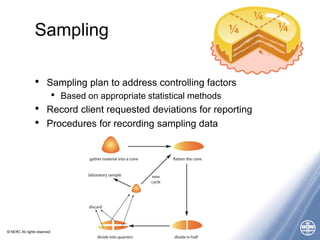
The document outlines quality management principles essential for laboratory practice, emphasizing the importance of a quality management system to ensure analytical reliability and customer trust. It discusses international standards such as ISO 17025, detailing management and technical requirements necessary for accreditation and demonstrating technical competence. Additionally, the document covers objectives for workshops aimed at addressing challenges faced by laboratories, particularly in Africa, while promoting best practices for data management and quality assurance.