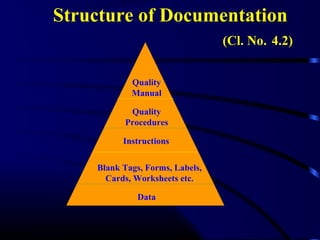
ISO/IEC 17025 outlines the requirements for laboratories to demonstrate competence and generate reliable test and calibration results. It covers management requirements like documentation, audits, and reviews as well as technical requirements for personnel, methods, equipment, measurement traceability and reporting. The standard requires laboratories to have a quality management system in place to ensure consistent, valid results and provide traceable measurements that can be compared internationally through an unbroken chain of calibrations.