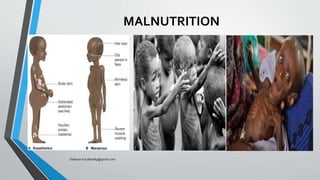
The document discusses the importance of following regulatory standards and ISO 17025, highlighting events like the thalidomide tragedy where lack of standards led to harmful effects. It covers the history and requirements of ISO 17025, including structural requirements for laboratories, resource requirements for personnel, facilities, equipment and traceability, as well as process requirements. The goal of standards is to ensure quality, safety and avoid issues that negatively impact consumers.