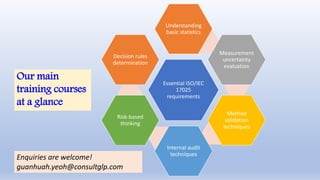
This document presents a variety of training courses designed to help participants meet ISO/IEC 17025:2017 requirements. The courses cover topics such as statistical methods, measurement uncertainty, method validation, internal audit techniques, and risk-based thinking, emphasizing hands-on learning and practical applications. Participants receive training materials, Excel worksheets, and post-training consultation to support their ongoing development.