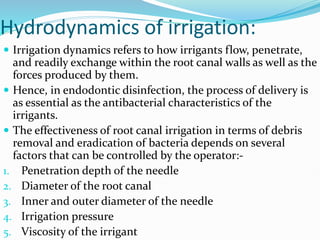
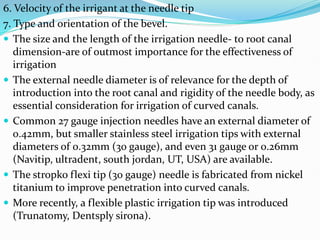
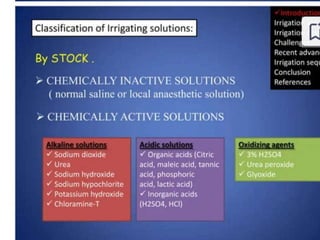
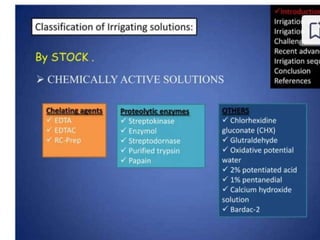
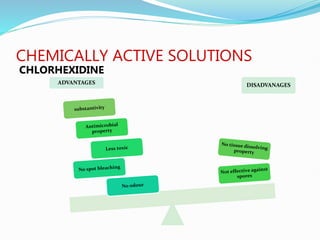
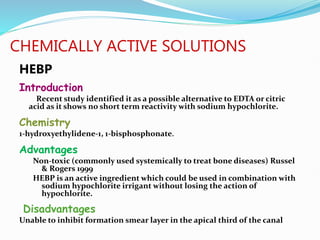
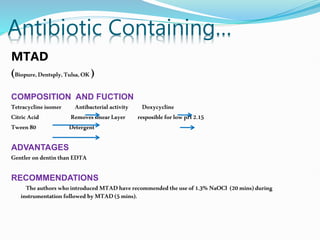
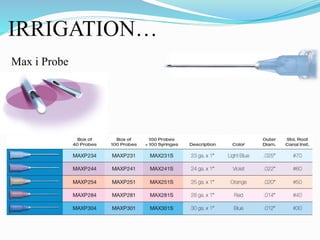
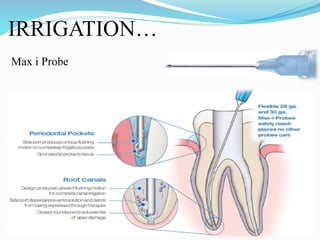
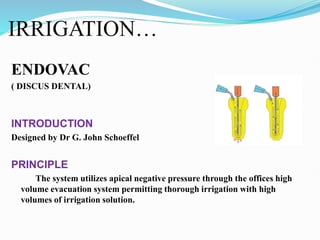
The document presents a comprehensive overview of irrigation in endodontics, detailing its history, objectives, ideal requirements for irrigating solutions, and various types of solutions used. It discusses the hydrodynamics of irrigation, the effectiveness of different chemical agents including sodium hypochlorite, chlorhexidine, and EDTA, highlighting their mechanisms, advantages, and disadvantages. Additionally, it covers factors affecting irrigation activity and the significance of irrigation in root canal treatment.