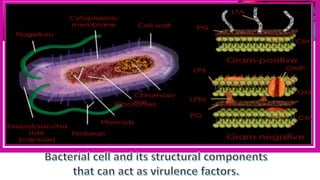
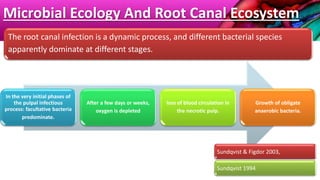
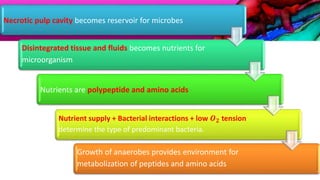
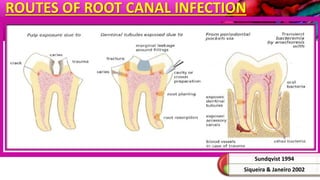
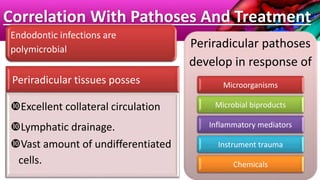
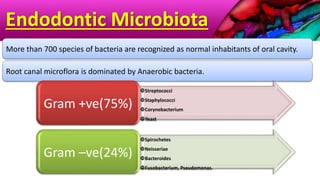
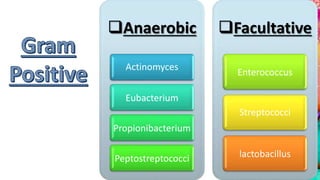
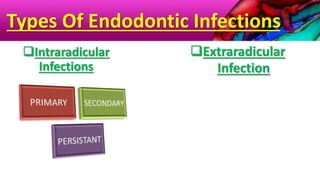
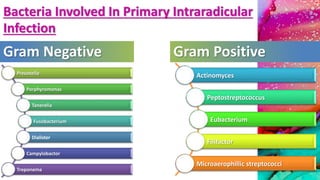
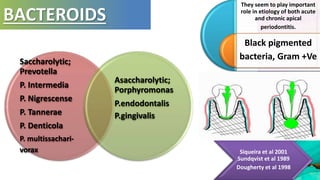
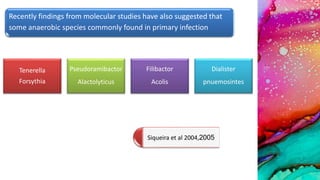
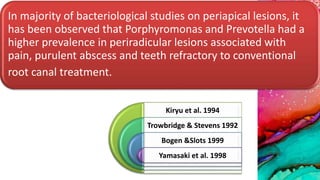
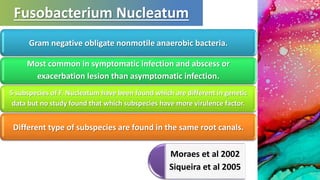
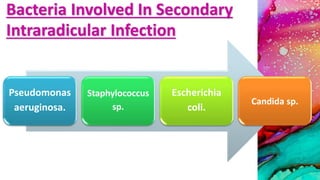
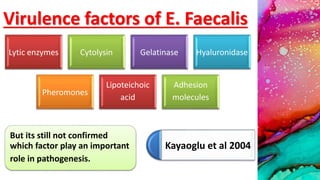
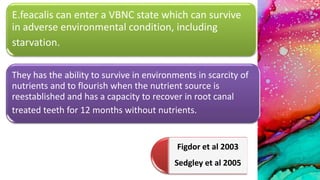
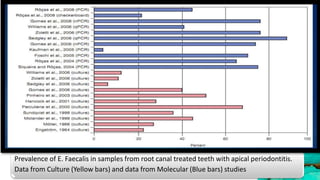
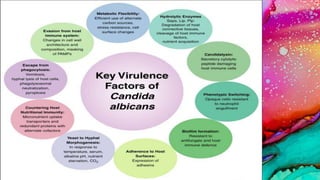
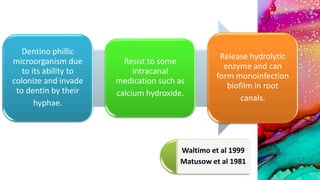
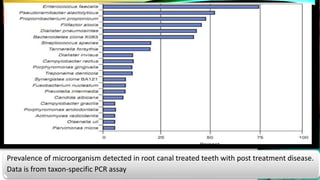
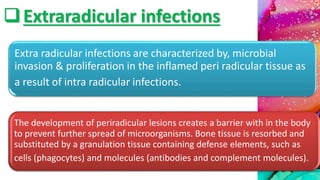
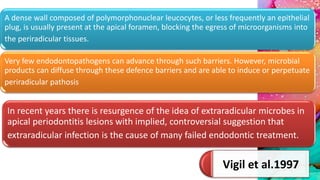
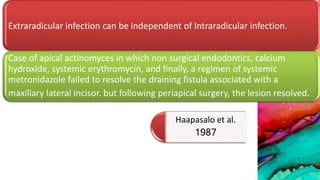
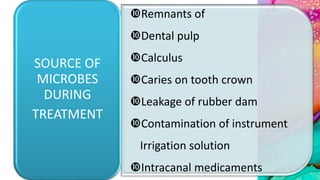
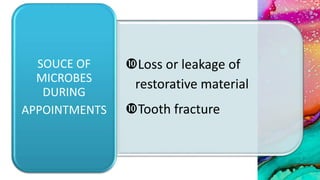
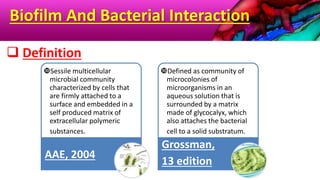
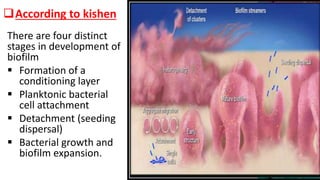
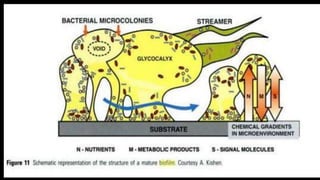
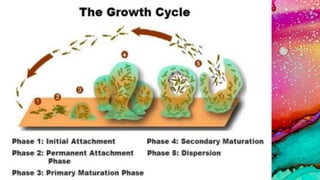
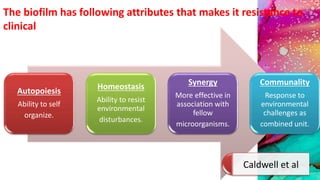
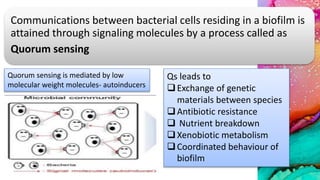
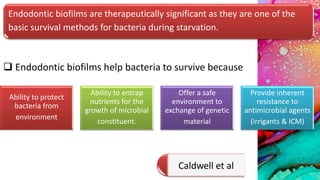
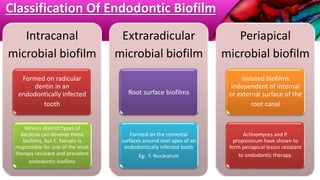
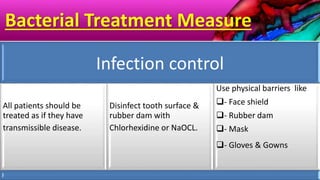
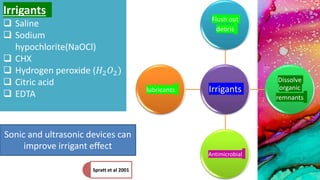
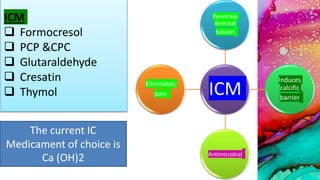
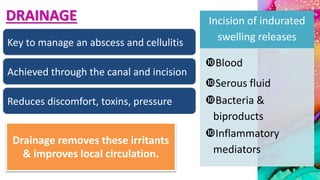
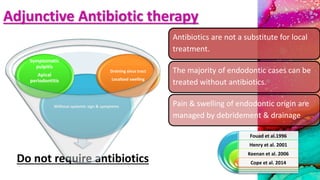
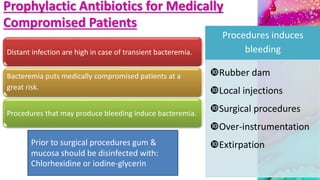
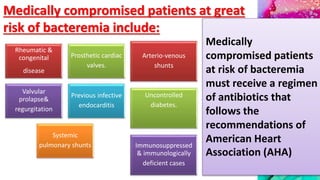
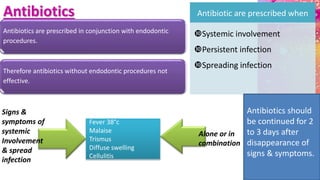
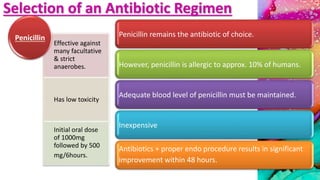
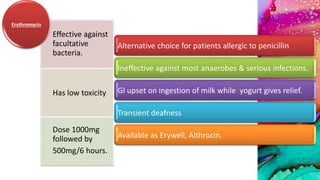
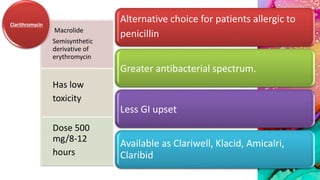
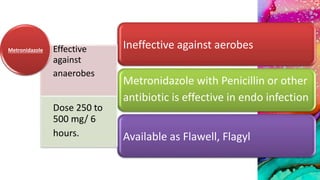
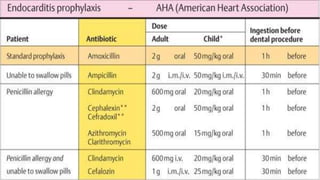
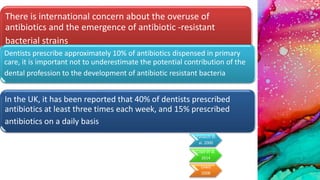
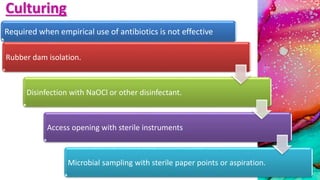
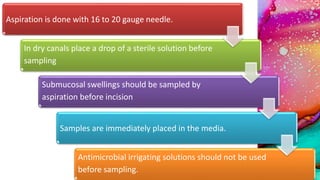
This document provides an overview of endodontic microbiology. It begins with an introduction to how microorganisms cause pulp and periradicular infections. It then discusses the mechanisms of microbial pathogenicity and virulence factors. It describes the microbial ecology of the root canal ecosystem and how the environment changes over time. It outlines the various routes of root canal infection and the typical microbes involved in primary, secondary, and persistent intraradicular infections. It also discusses extraradicular infections. The document covers biofilm formation and bacterial interactions. It concludes with discussing treatment measures and references.