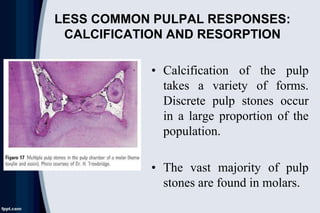
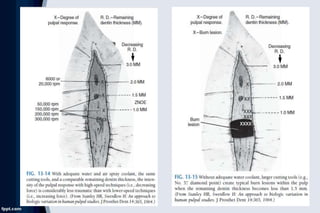
The document discusses pulp pathology and its sequelae. It covers the response of the pulp to dental caries, including immune response, hard tissue response to irritation, and histologic changes in acute and chronic inflammation. It also discusses neural changes during pulpal inflammation, antiinflammatory mechanisms, less common responses, iatrogenic effects, systemic factors, and pulpal sequelae to trauma. Causes of pulp inflammation, necrosis, and dystrophy include bacterial, traumatic, iatrogenic, chemical, and idiopathic factors.