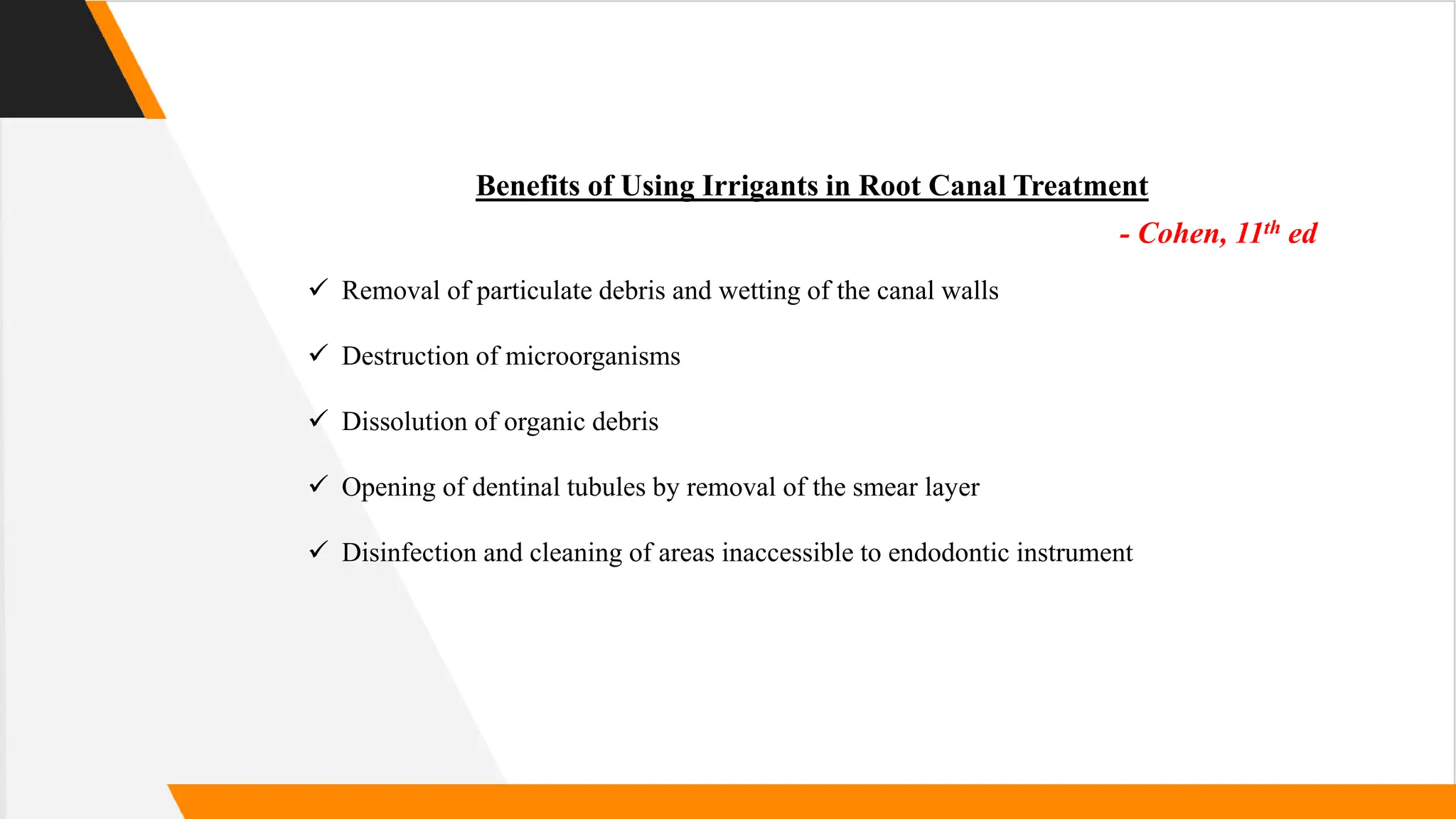
The document provides a comprehensive overview of root canal irrigants, discussing their history, properties, classification, and various types such as sodium hypochlorite and EDTA. It emphasizes the importance of effectively irrigating root canals to enhance endodontic treatment outcomes by removing debris and microorganisms. Additionally, it explores irrigation dynamics, techniques, and the biological and mechanical objectives of using different chemical solutions in dental procedures.



































































































































![ETHYLENEDIAMINE TETRA-ACETIC ACID (EDTA)
• It is a polyaminocarboxylic acid with the formula
[CH2N(CH2CO2H)2]2.
• It is usually used in a concentration between 10%- 17%, and to
increase its chelating effectiveness, its pH was modified from 4
to a value between 7 and 8
• The optimal pH for the demineralizing efficacy of EDTA on
dentin was shown by Valdrighi to be between 5.0 and 6.0.](https://image.slidesharecdn.com/5-240328174225-0ba6aa04/75/VARIOUS-ROOT-CANAL-IRRIGANTS-IN-ENDODONTICS-133-2048.jpg)














































![• A 3.8% w/v silver diamine fluoride (Ag[NH3]2F) solution has been developed for
intracanal irrigation..
SILVER DIAMINE FLUORIDE](https://image.slidesharecdn.com/5-240328174225-0ba6aa04/75/VARIOUS-ROOT-CANAL-IRRIGANTS-IN-ENDODONTICS-180-2048.jpg)






























![• NB water was produced by using a nanobubble generator, Foamest (Nac Corp, Seki, Japan).
• The NB generator consisted of a casing for supplying pressurized gas, a nanoporous
polypropylene film (MONOTORAN film [Nac Corp]), and water flowing outside them.
• The film had several nanometers pores that allow gas to pass through
• The gas ejected from the film is crushed by the spreading force of water flowing outside the film,
which eventually leads to the formation of NB water](https://image.slidesharecdn.com/5-240328174225-0ba6aa04/75/VARIOUS-ROOT-CANAL-IRRIGANTS-IN-ENDODONTICS-211-2048.jpg)


























