Embed presentation





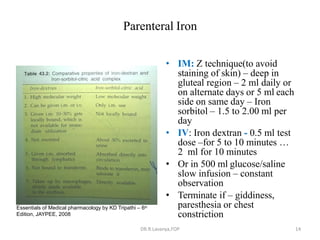
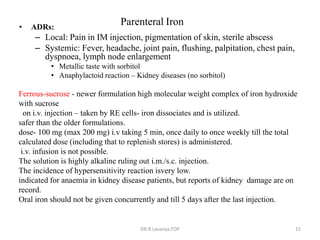





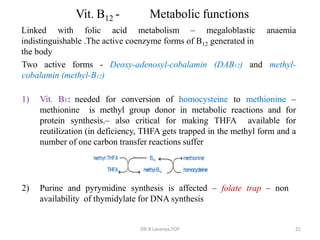
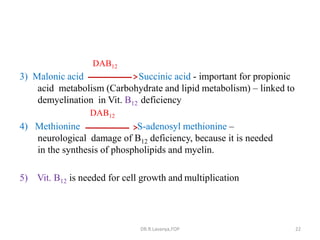







This document discusses haematinics, which are substances required for blood formation used to treat anaemias. It describes different types of anaemias caused by blood loss, impaired cell formation due to deficiencies in iron, vitamin B12, or folic acid, or increased destruction of red blood cells. It provides details on iron including absorption, transport, storage, requirements, sources, and preparations for both oral and parenteral administration. It also discusses acute iron poisoning treatment and mentions that vitamin B12 and folic acid deficiencies can result in megaloblastic anaemia.


































