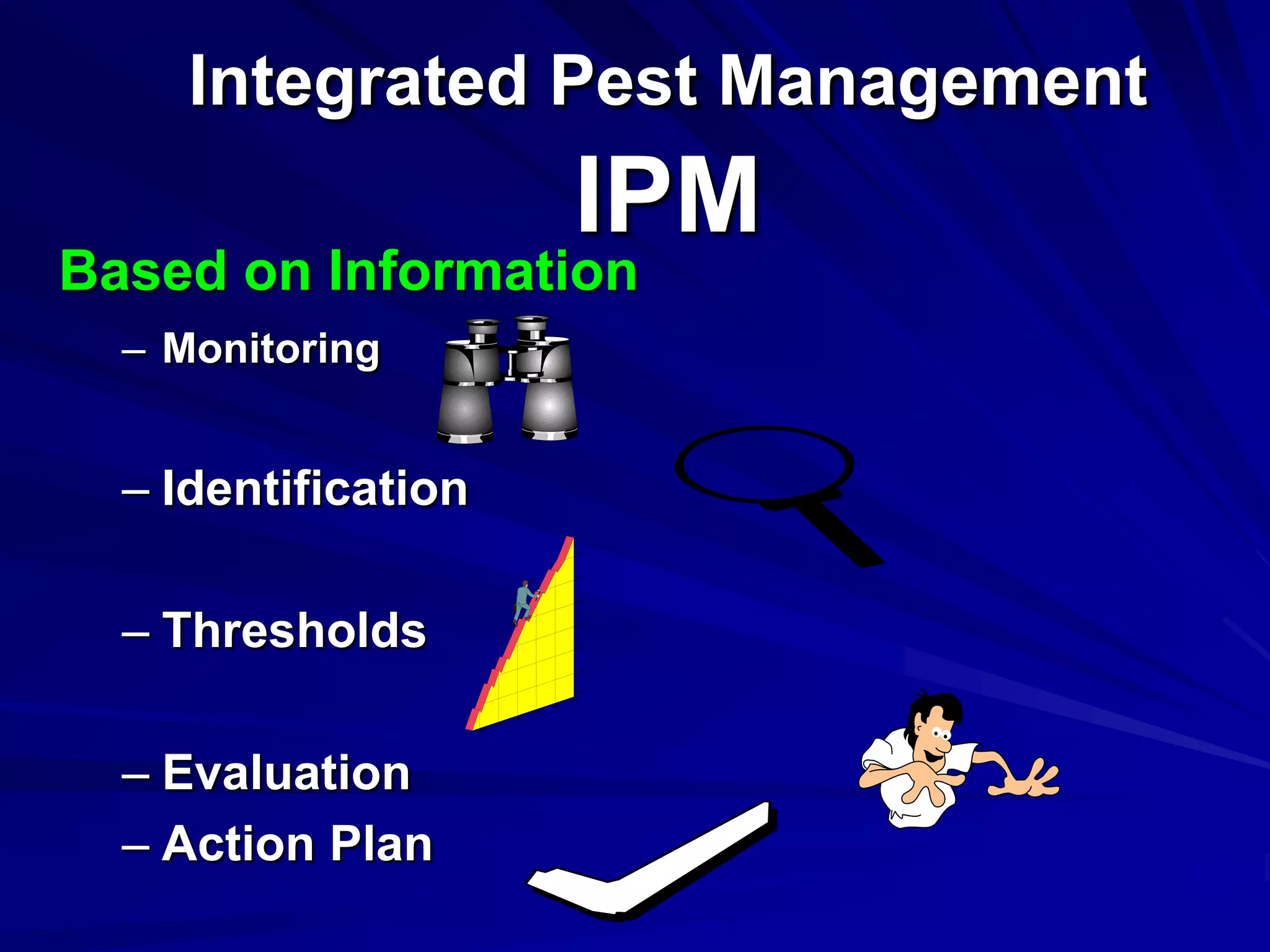
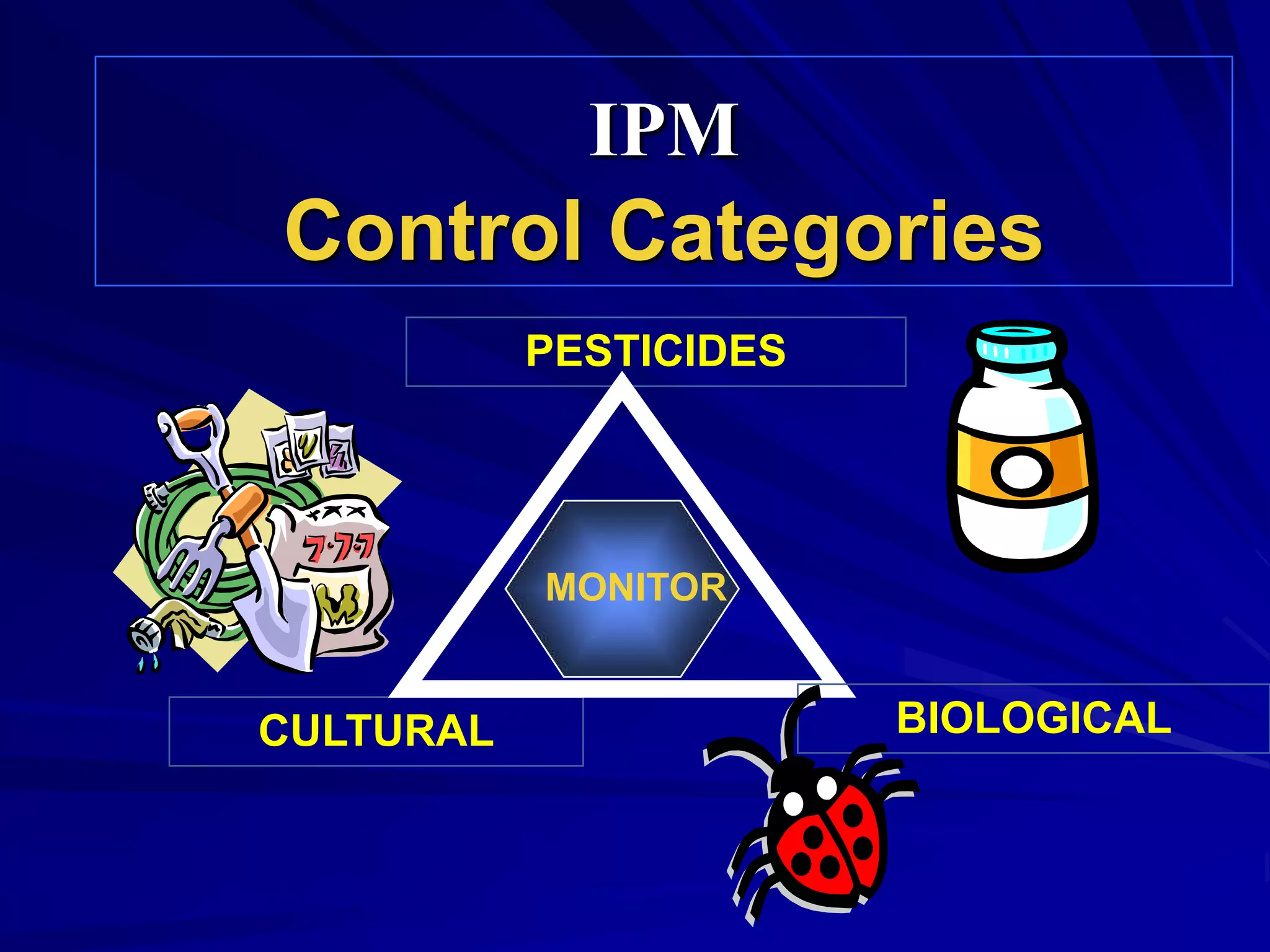
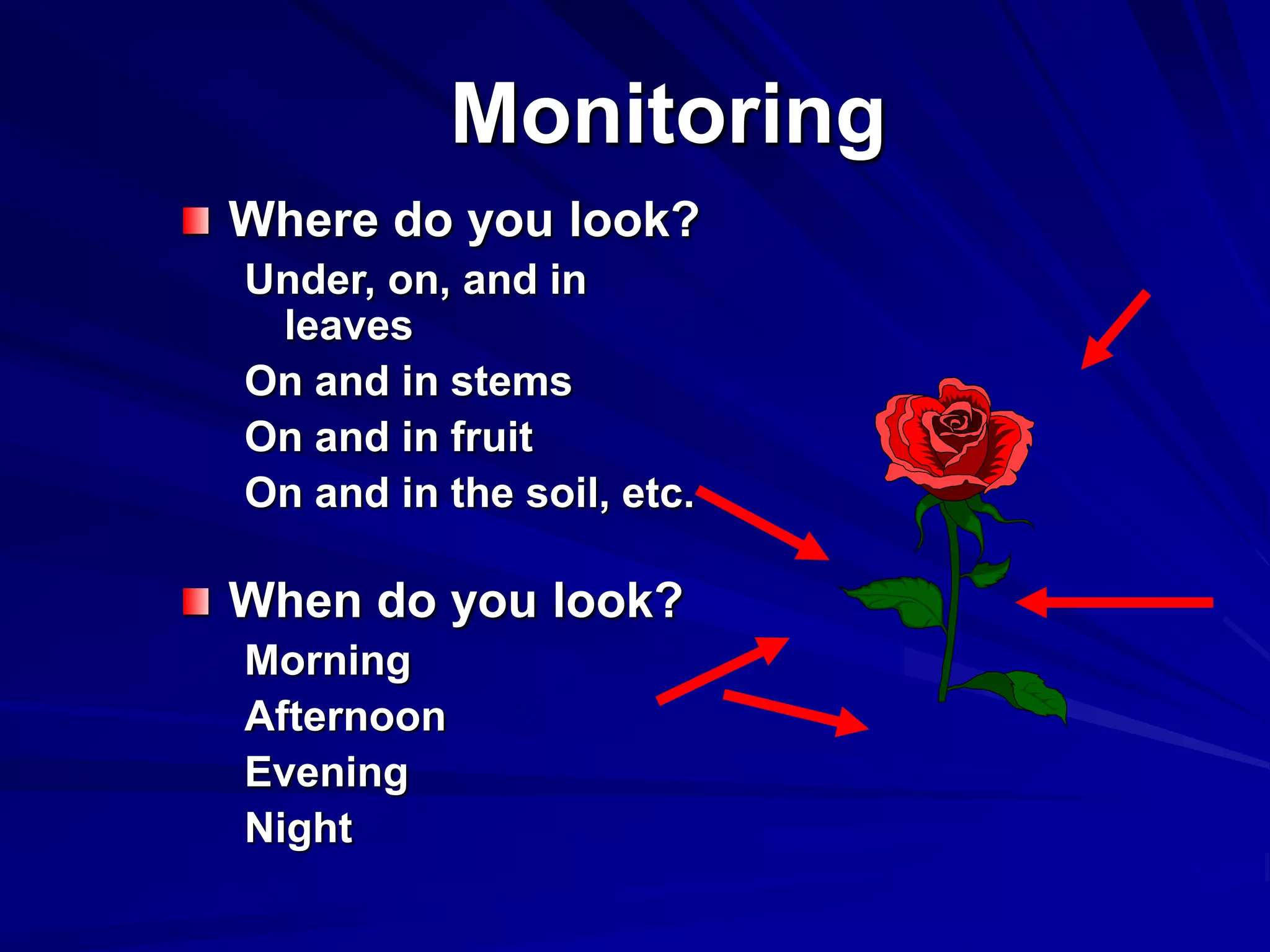
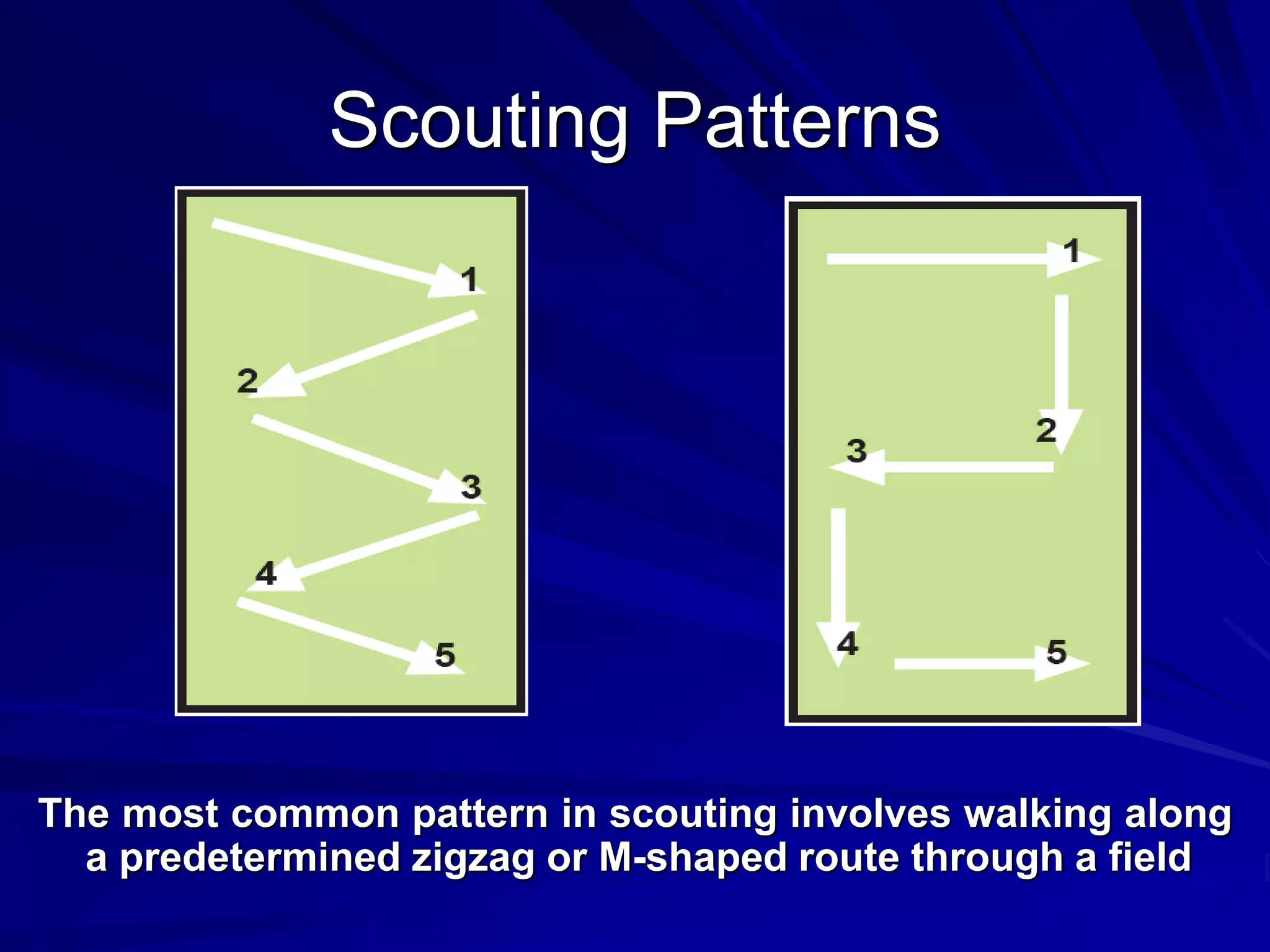
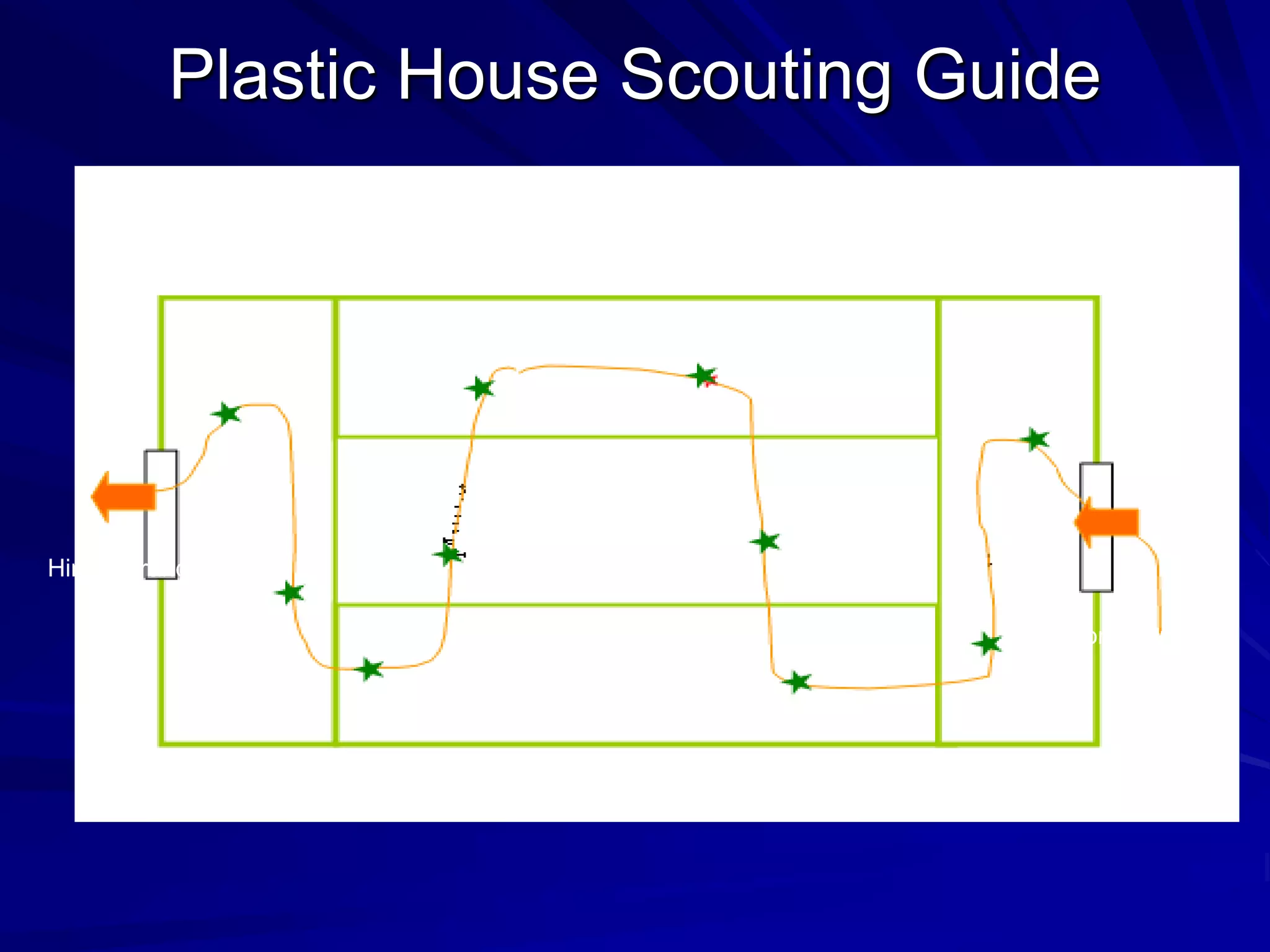
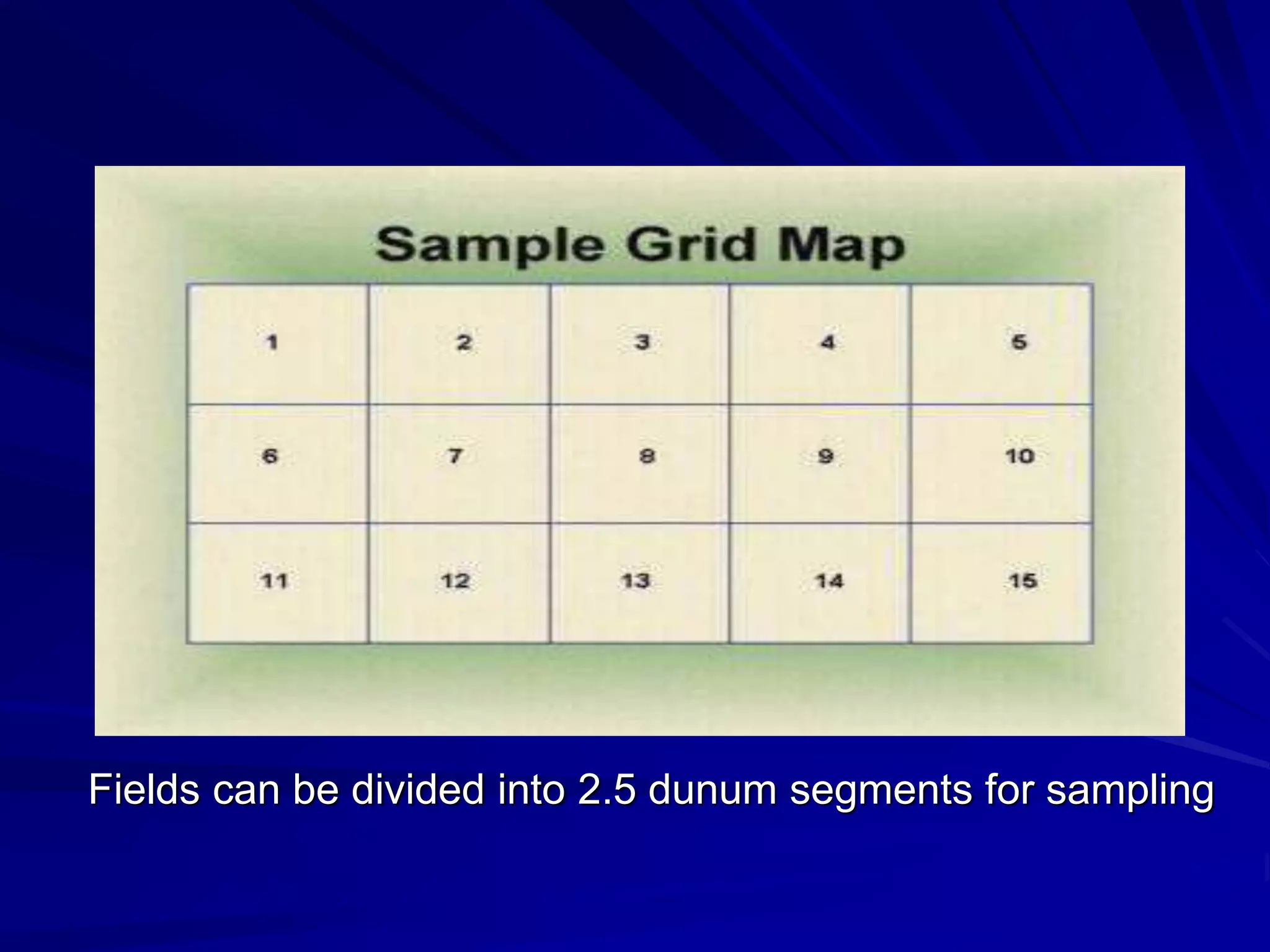
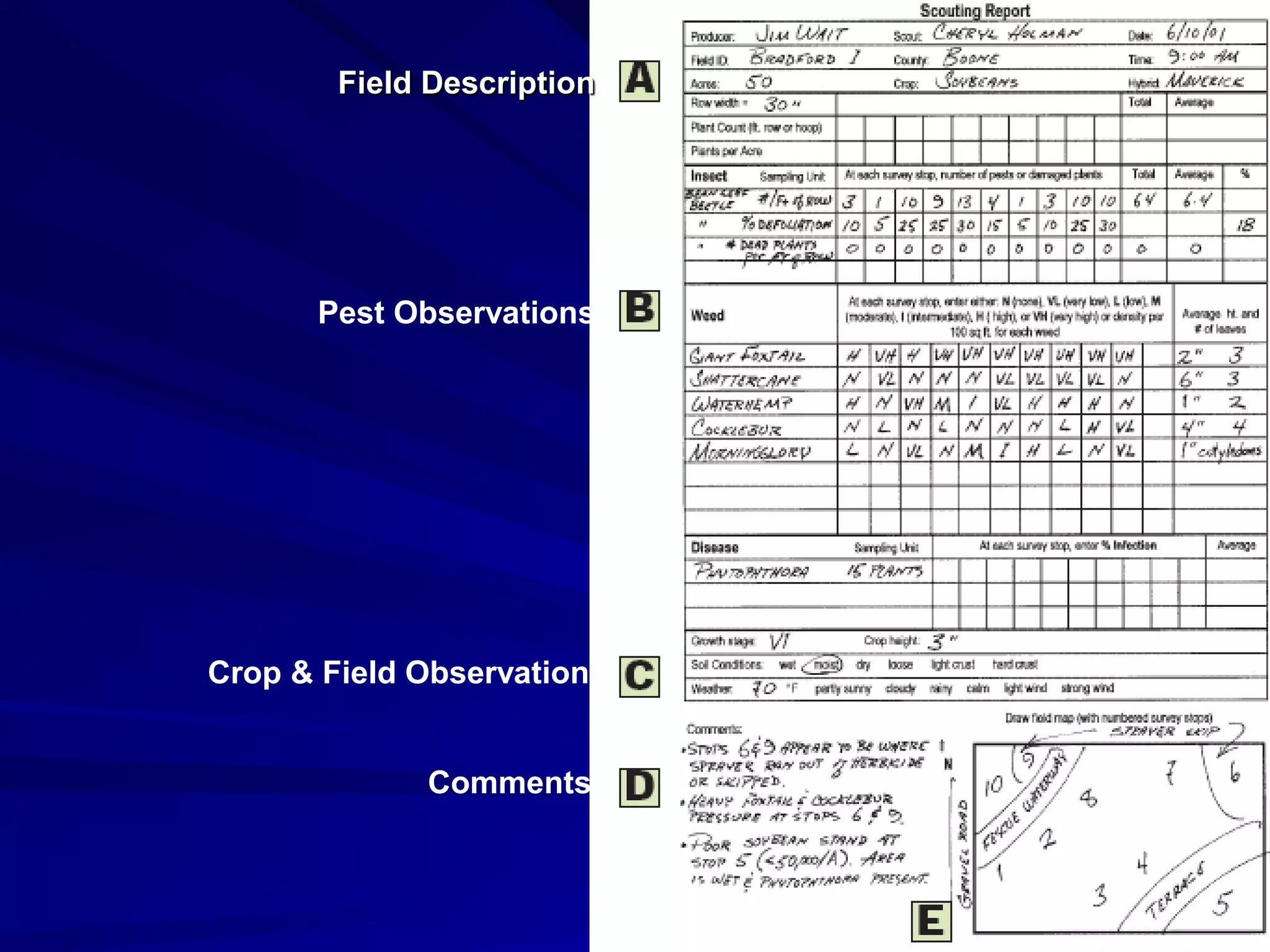
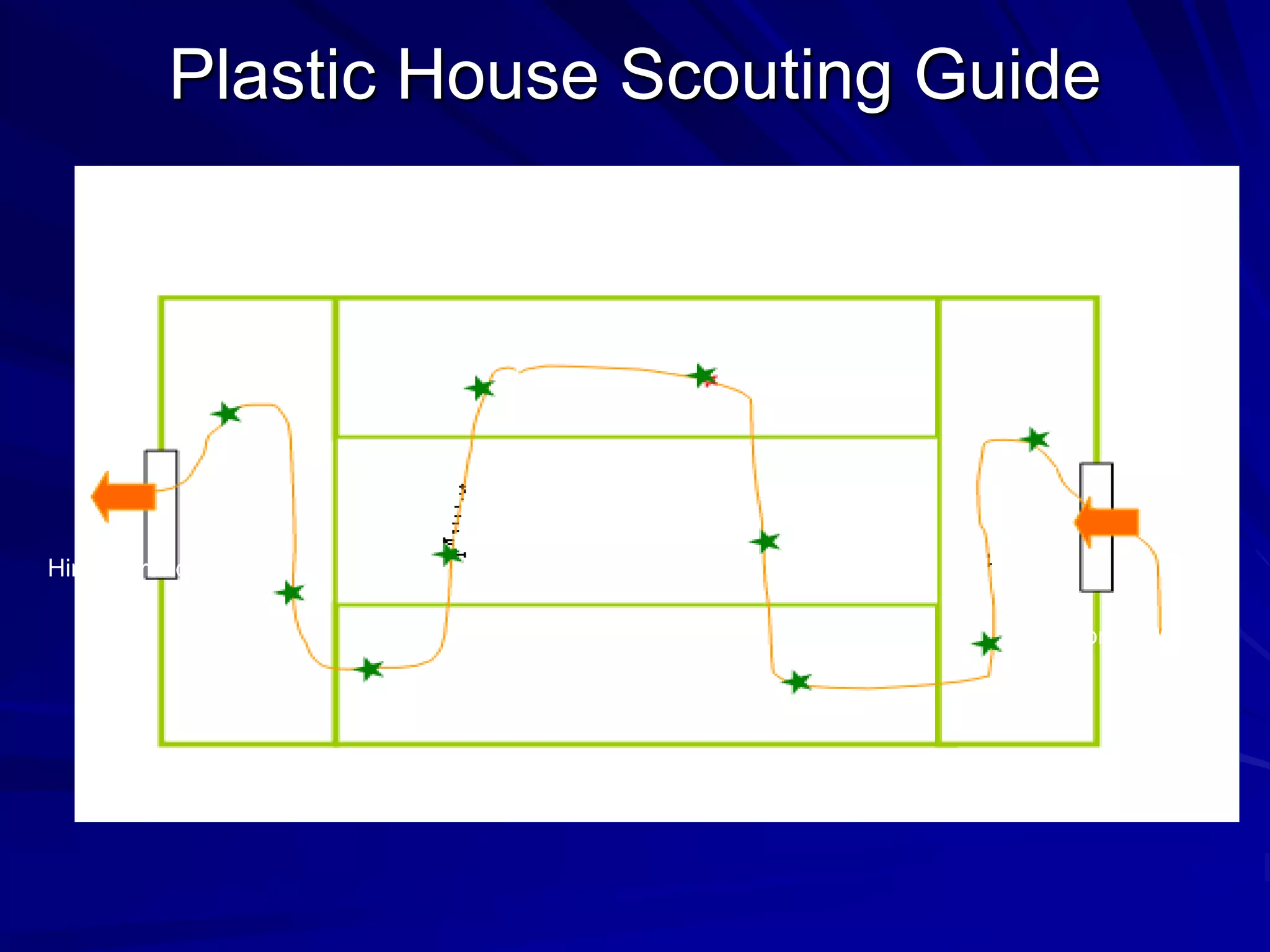
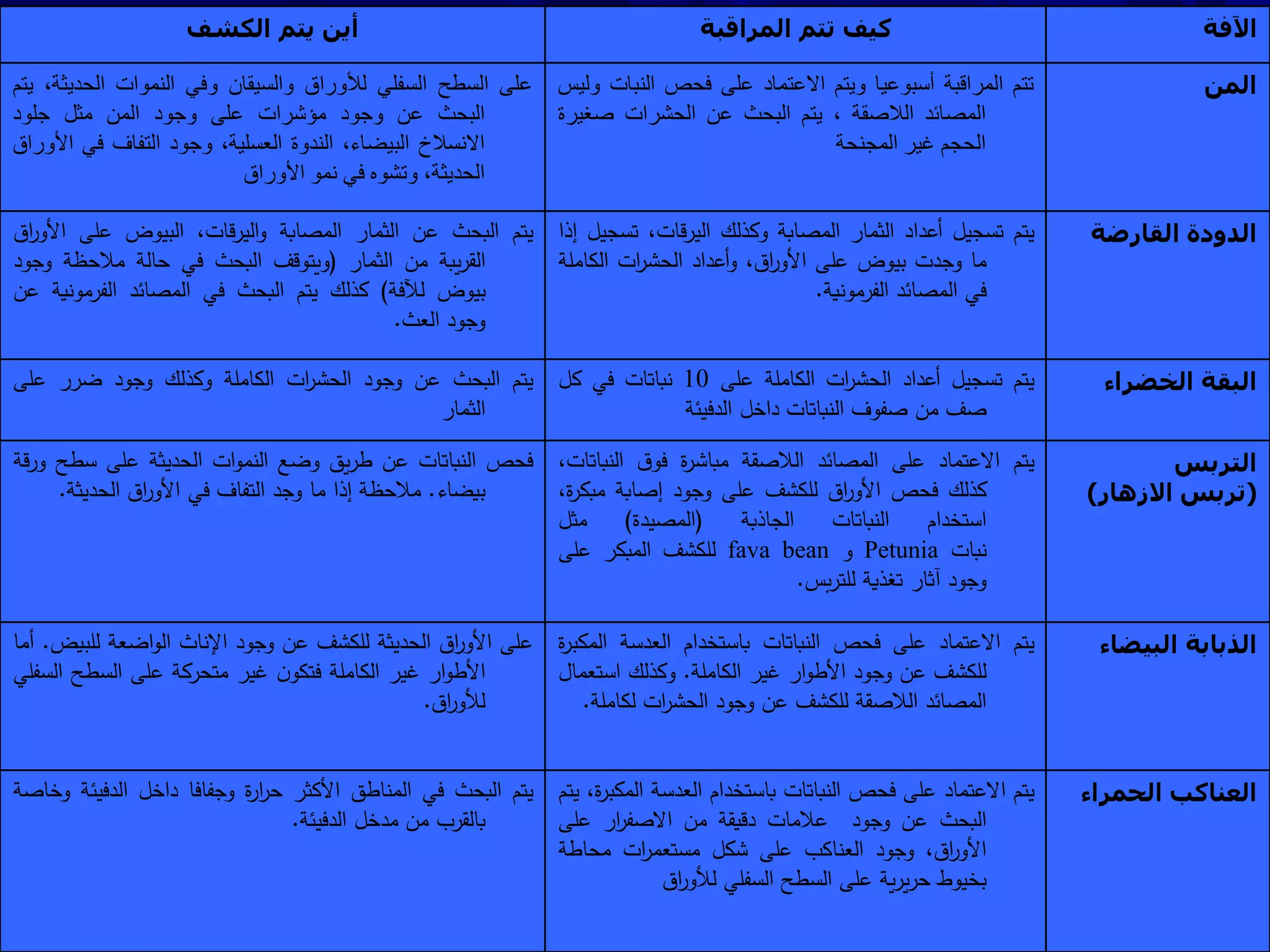
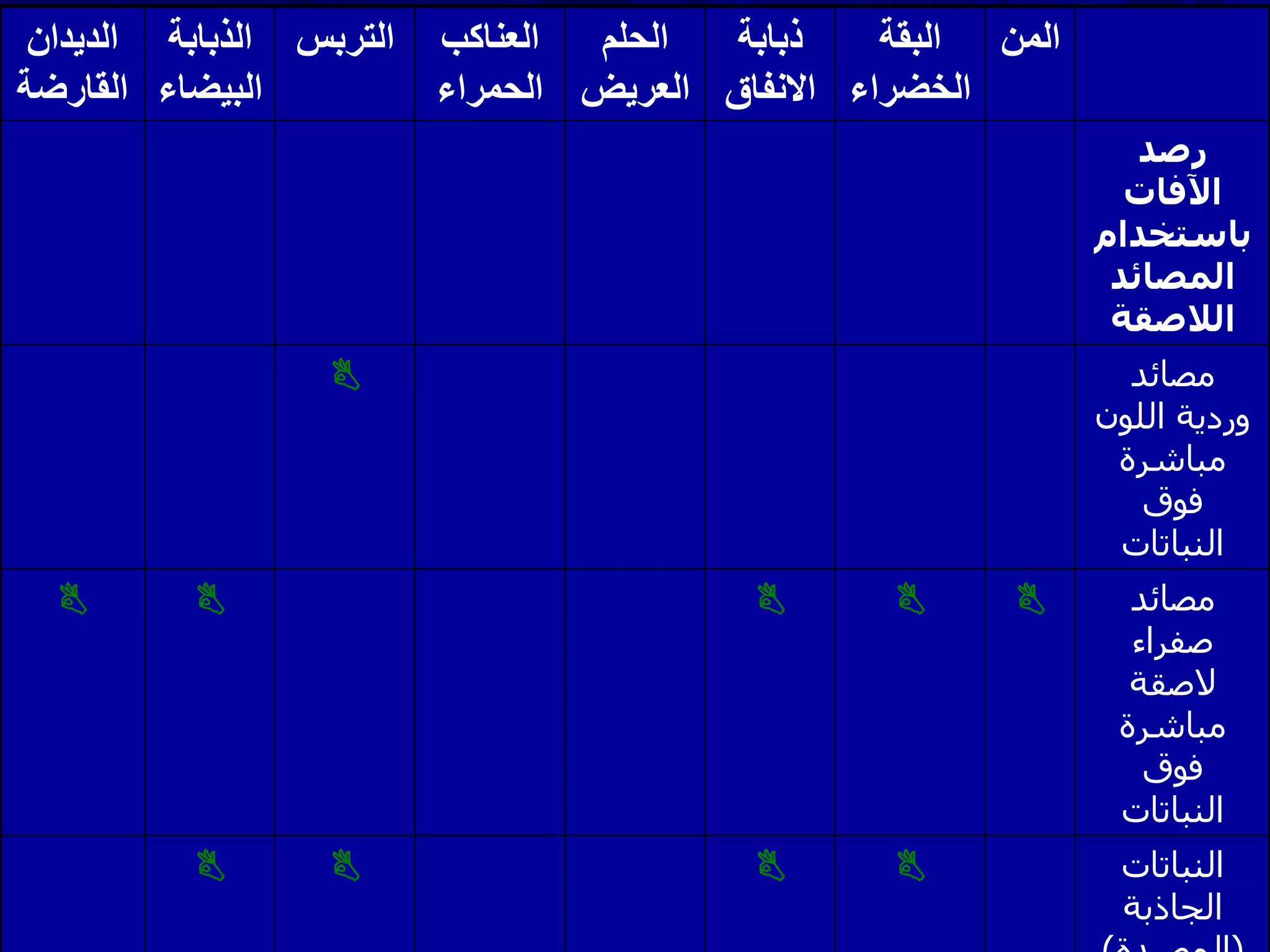
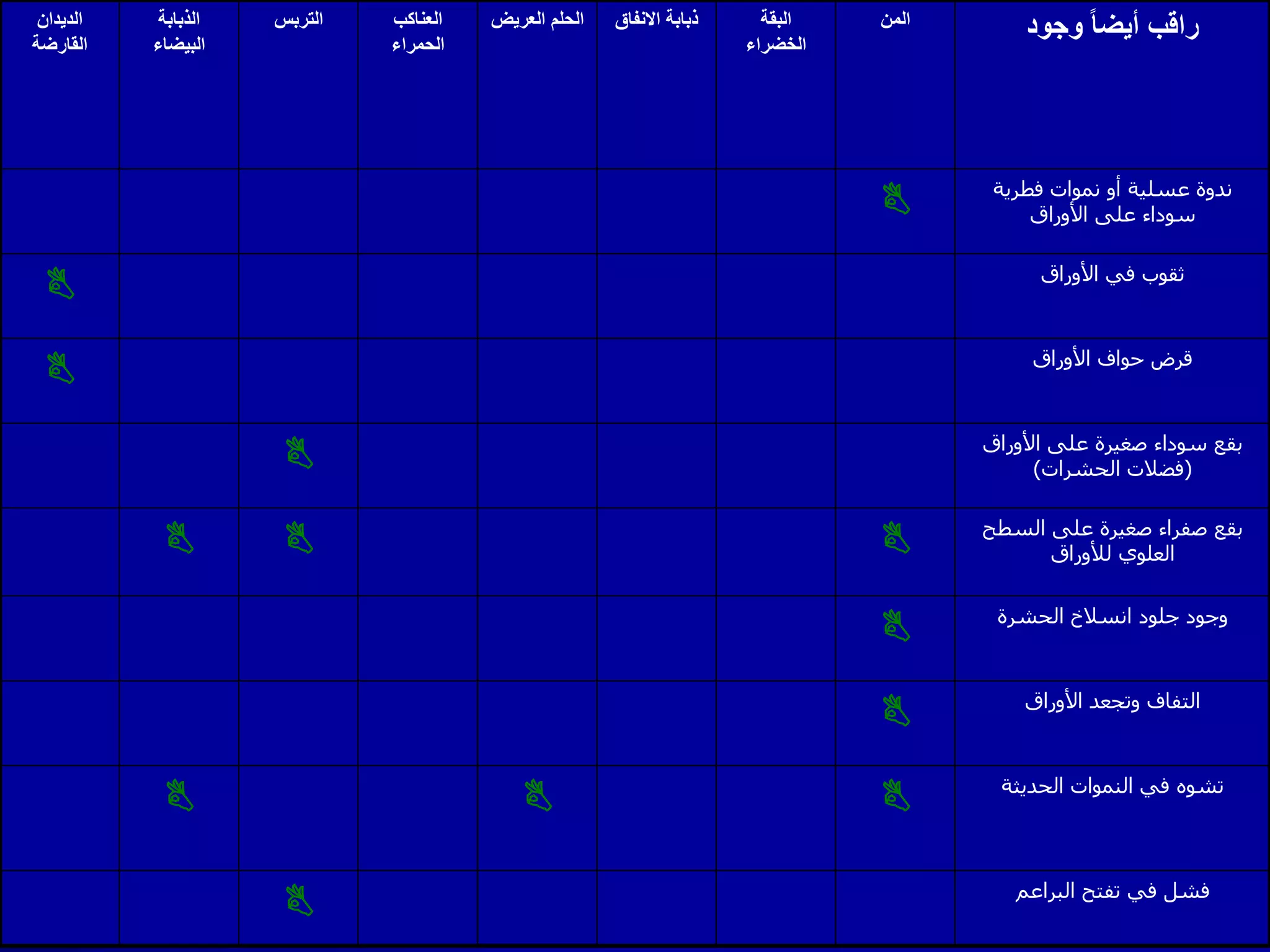
Monitoring is a key part of integrated pest management. It involves regularly surveying crops to identify any pests, diseases, or weeds. This helps determine if control measures are needed. Monitoring methods include direct observation of pests on plants, using beating sheets to dislodge pests from plants, sweeping for insects, and trapping insects. Records of monitoring activities including the type and number of pests found help inform pest management decisions.