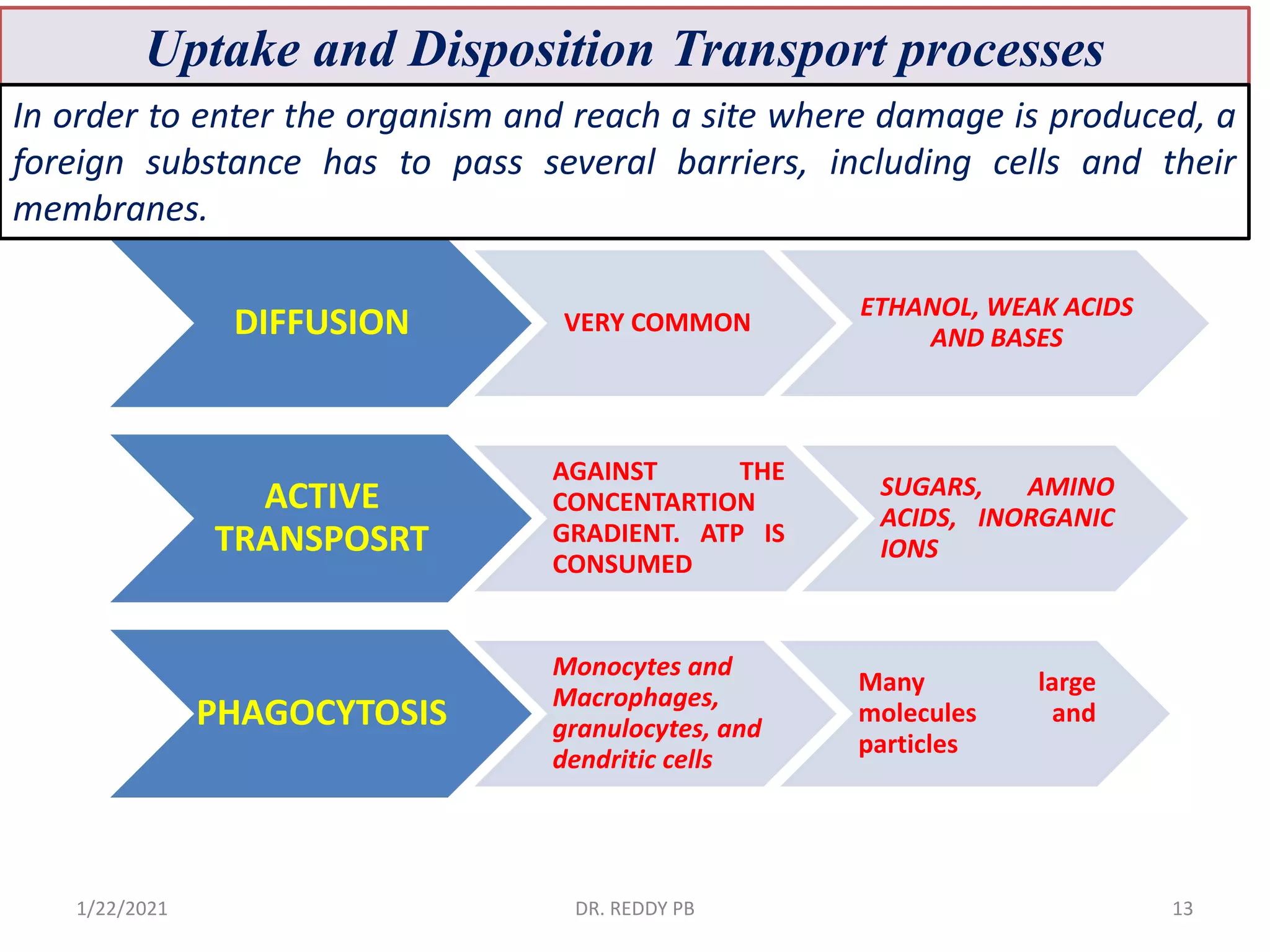
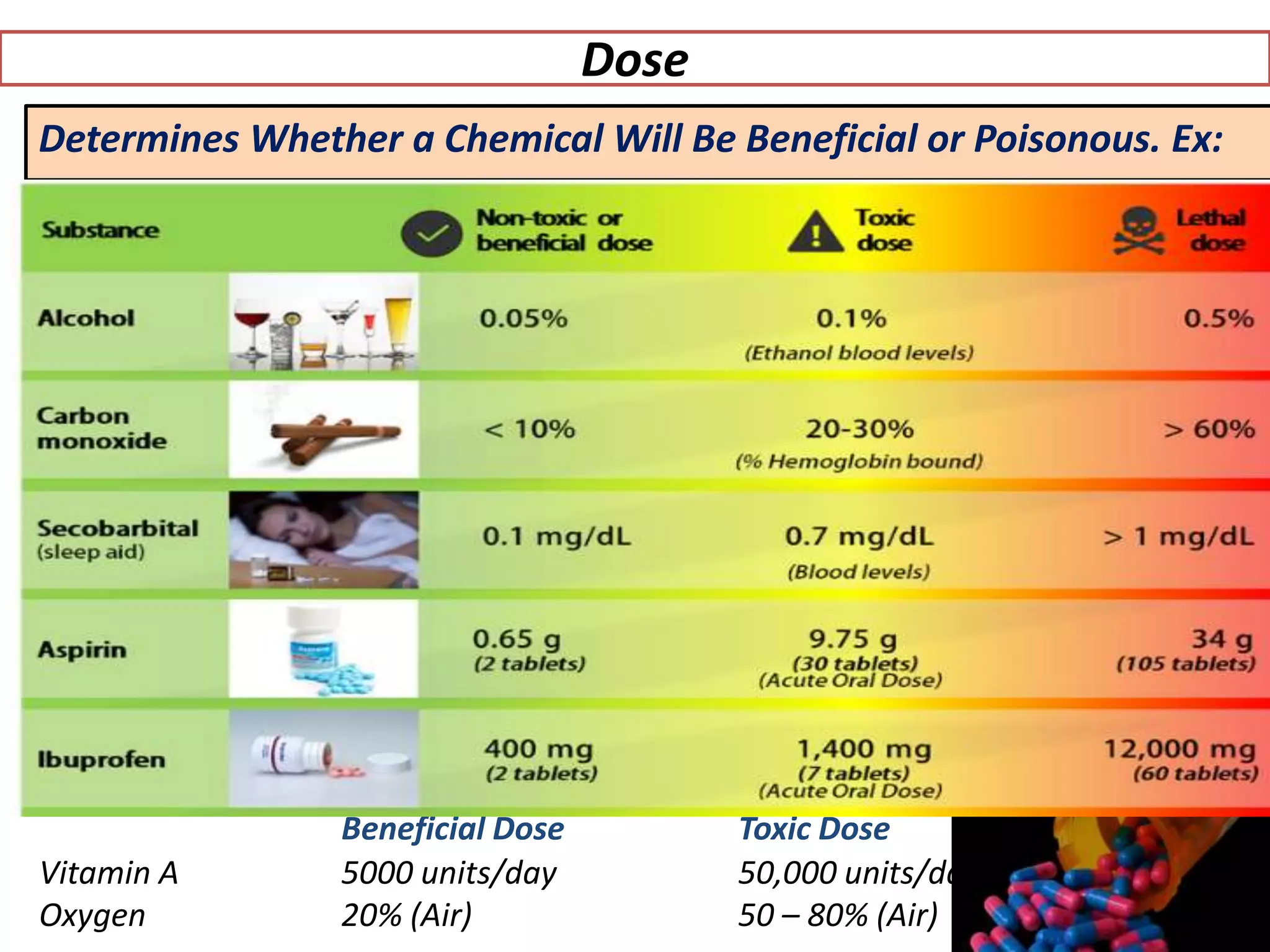
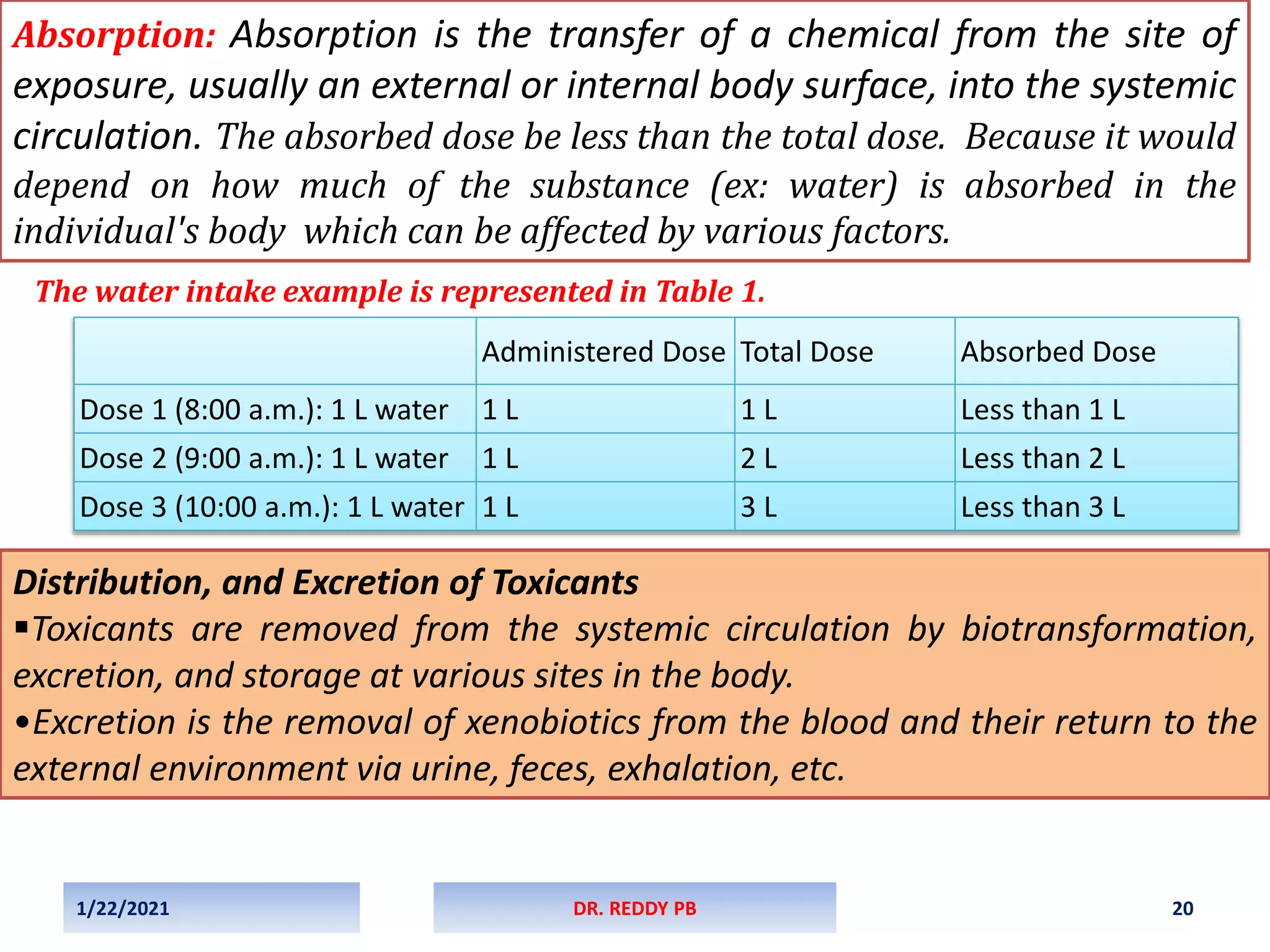
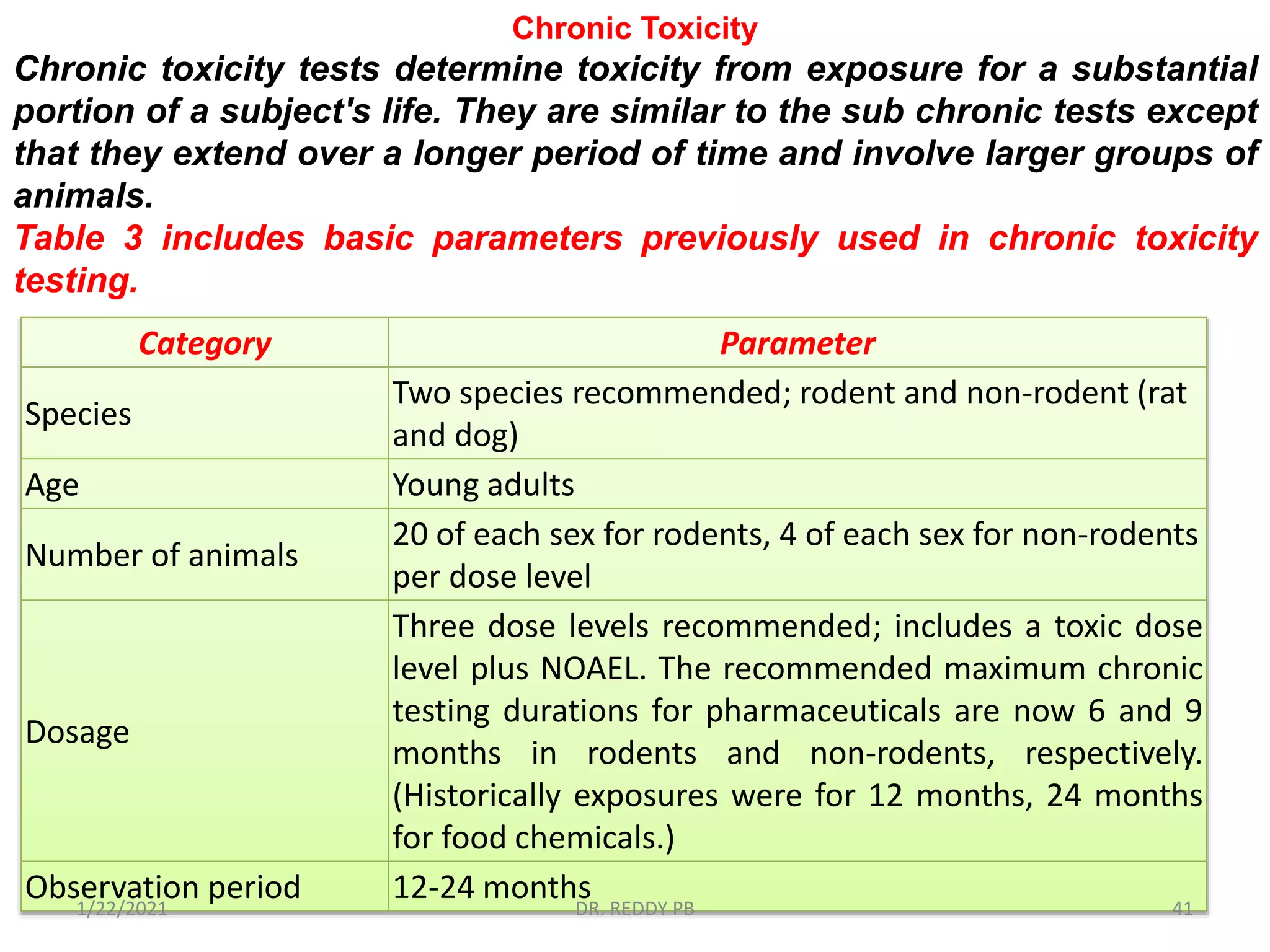
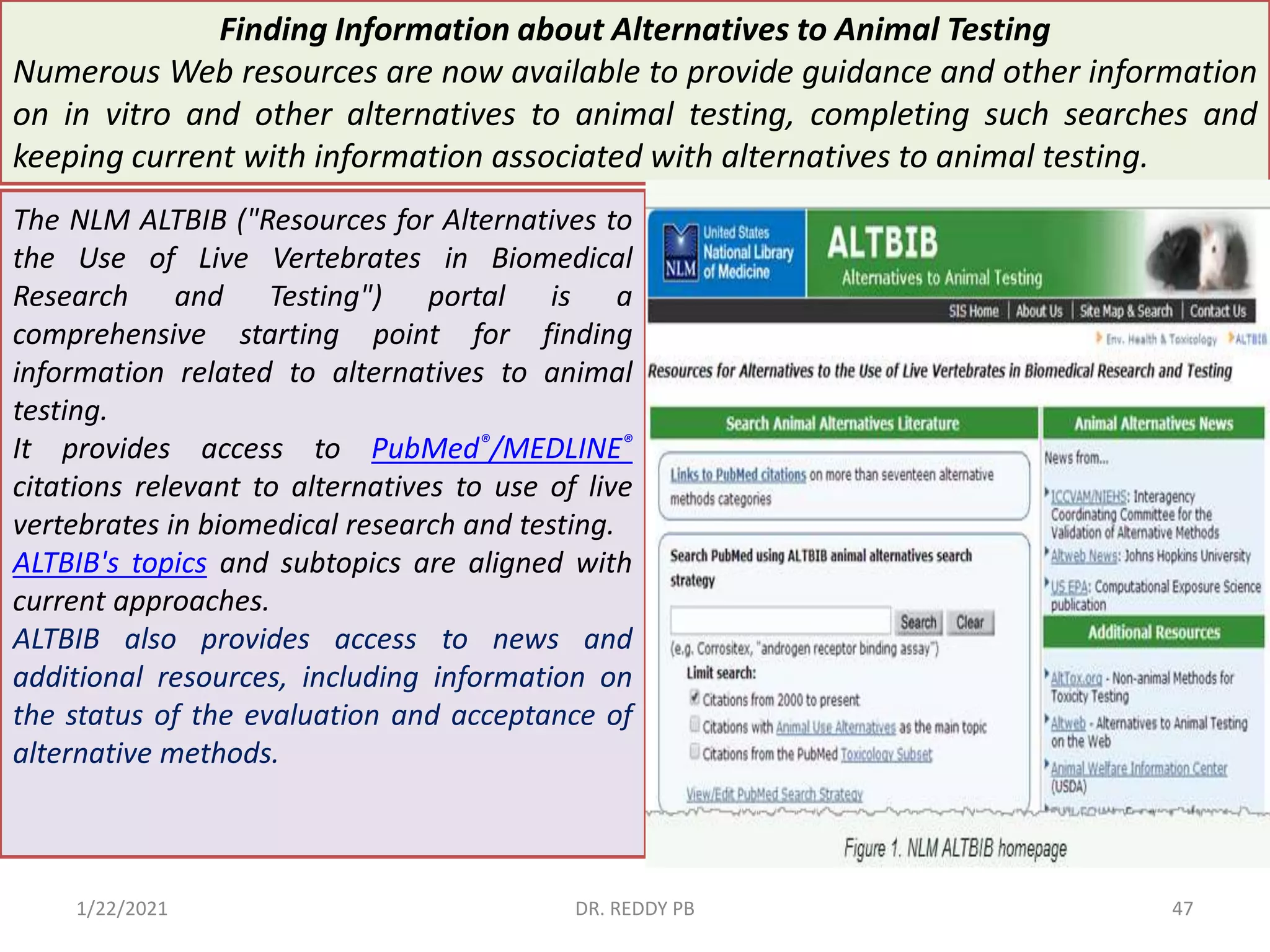
The document provides an overview of toxicology, detailing its principles, history, dose-response relationships, and toxicity testing methods. It defines key concepts such as toxicity, xenobiotics, and risk assessment, while exploring the effects of dose, exposure routes, and absorption in biological systems. The educational content aims to advance the understanding of chemical safety and the evaluation of toxic substances effects on organisms.