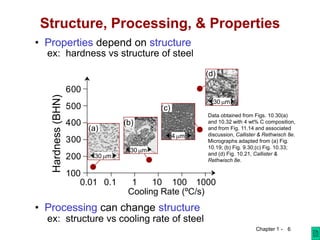
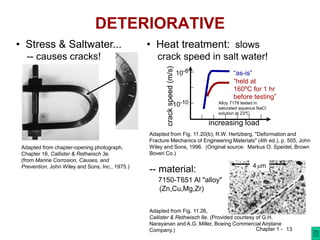
This course introduces students to modern materials engineering topics including material structure, how structure dictates properties, and the impact of materials on society. Students will learn about materials selection processes and how processing can change a material's structure and properties for different applications. The goals are for students to properly use materials, recognize new design opportunities using materials selection, and understand the relationships between a material's properties, structure, and processing.