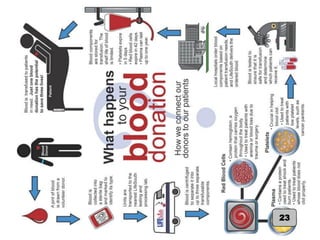
This document provides an introduction to blood transfusion. It begins with definitions of key terms like blood bank and blood donor. It then provides a brief history of blood banking, noting important developments like the use of anticoagulants and refrigerated storage. The document outlines the collection and processing of whole blood into components like red blood cells, plasma, platelets and cryoprecipitate. It discusses the storage and management of these blood products, including shelf lives and temperatures. It also defines red blood cell storage lesion and discusses efforts to minimize its effects. In summary, the document introduces students














![RBC STORAGE LESION
• Insufficient transfusion efficacy can result from red blood cell
(RBC) blood product units damaged by so-called storage lesion
[a set of biochemical and biomechanical changes which occur
during storage].
• With red cells, this can decrease viability and ability for tissue
oxygenation.
• Although some of the biochemical changes are reversible after
the blood is transfused, the biomechanical changes are less so,
and rejuvenation products are not yet able to adequately reverse
this phenomenon. 20](https://image.slidesharecdn.com/introductiontobloodtransfusion-180211190012/85/Introduction-to-blood-transfusion-21-320.jpg)