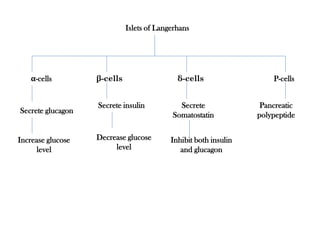
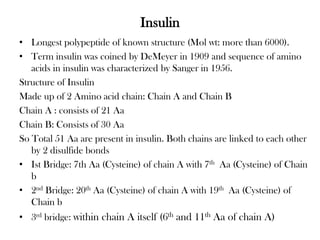
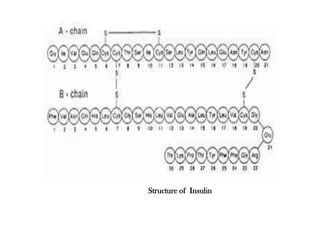
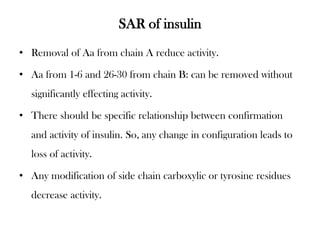
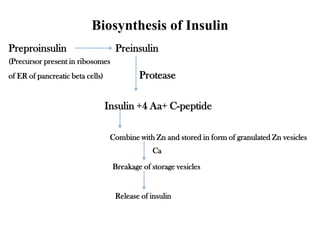
Insulin is a polypeptide hormone produced by the beta cells of the pancreas that regulates blood glucose levels. It controls carbohydrate, fat, and protein metabolism. Diabetes occurs when the pancreas does not produce enough insulin or the body does not properly use the insulin. The two main types of diabetes are type 1, where the pancreas does not produce insulin, and type 2, where the body is resistant to or does not properly use insulin. Both types can lead to complications if not controlled.