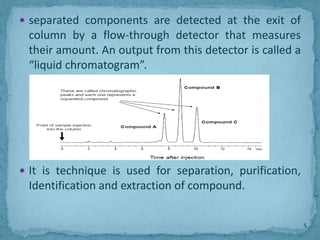
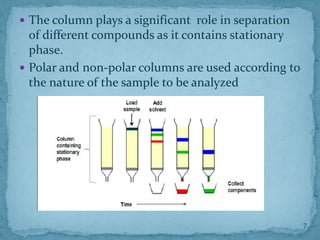
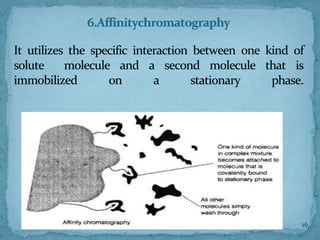
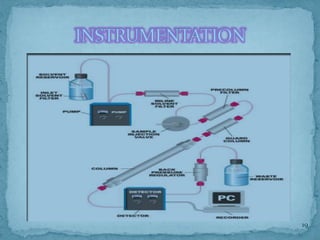
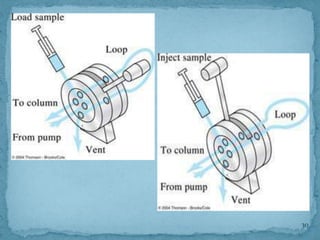
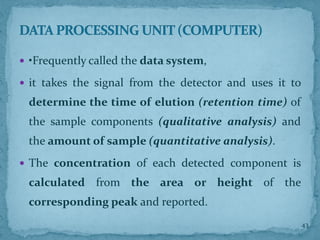
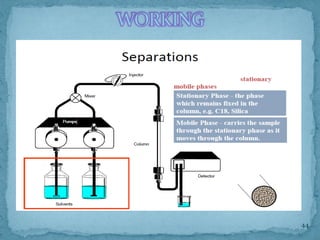
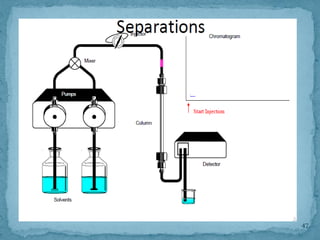
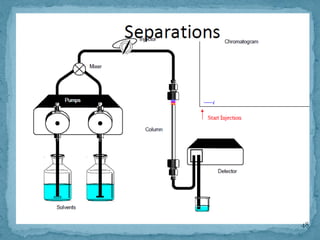
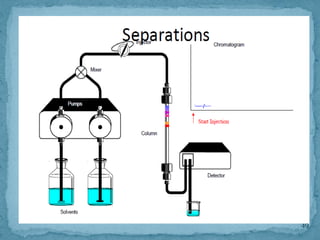
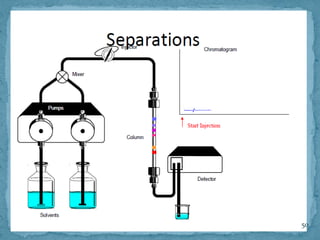
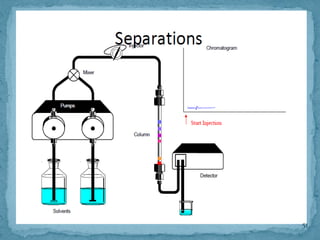
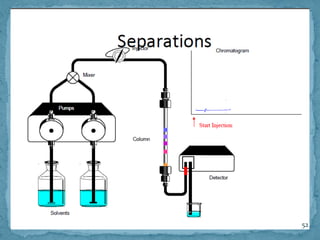
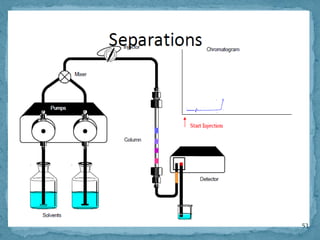
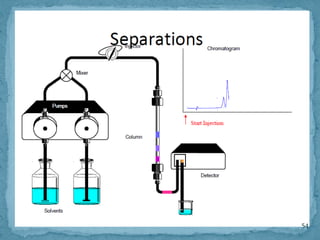
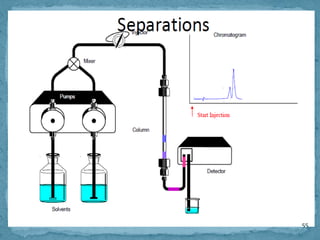
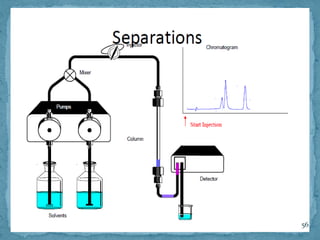
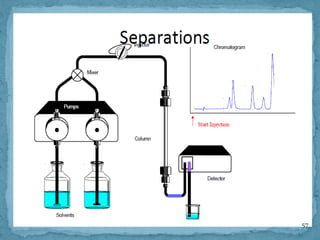
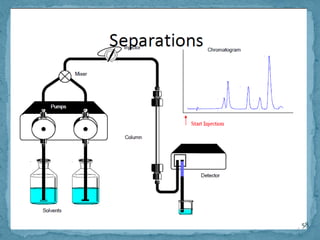
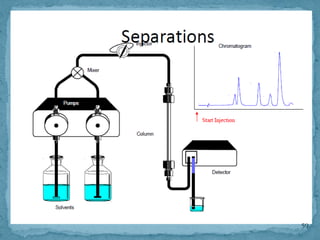
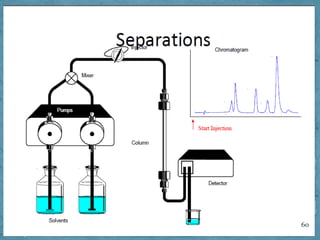
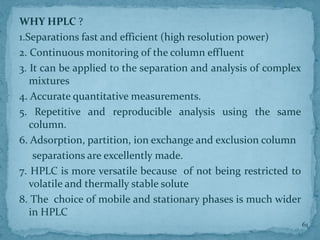
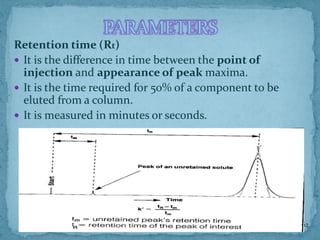
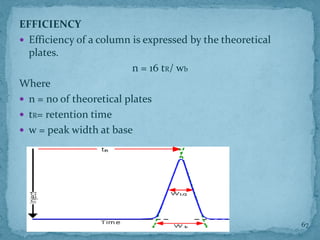
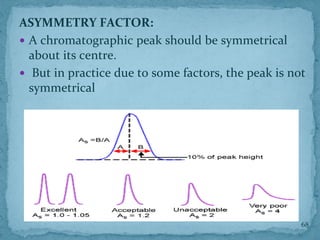
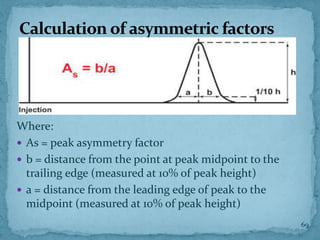
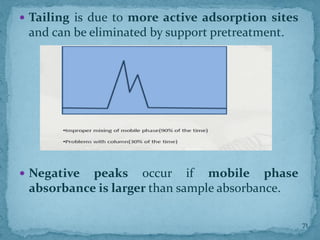
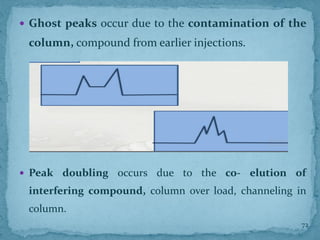
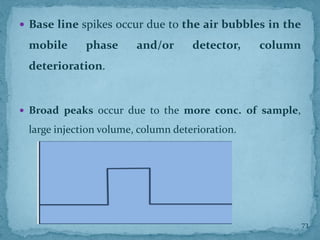
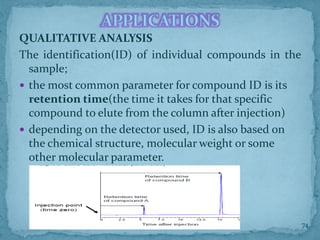
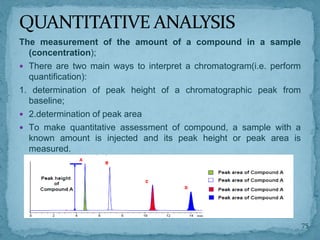
HPLC is a form of liquid chromatography that can separate compounds dissolved in solution. It works by injecting a sample into a column packed with tiny particles, then using a pump to force a liquid mobile phase through the column. This carries the sample components along the column at different speeds based on their interaction with the stationary phase, separating them. HPLC can separate a wide range of compounds and is used in pharmaceutical and chemical analysis applications.