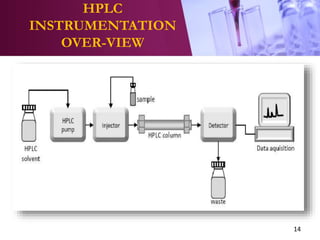
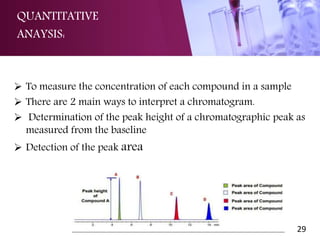
The document provides an overview of high performance liquid chromatography (HPLC). It begins with defining HPLC and explaining the basic principles of chromatography. It then describes the different types of HPLC based on the mode and principle of separation. The document also discusses HPLC instrumentation, including the solvent reservoir, pump, injector, column, and various detectors. It concludes by outlining some common applications of HPLC and discussing quantitative and qualitative analysis.













![1.Solvent reservoir & Mixing unit
& degassing system :
The appropriate solvents [mobile phase] from the reservoirs
are allowed to enter the mixing chamber where a homogenous
mixture is obtained.
Several gases are soluble in organic solvents.
When solvents are pumped under high pressure, gas
bubbles are formed which will interfere with the separation
process, steady base line and the shape of the peak.
Hence degassing of solvent is important. This can be done by
Vacuum filtration,
Ultrasonication
15](https://image.slidesharecdn.com/royalppthplc-181213071305/85/Royal-ppt-hplc-15-320.jpg)



![4. Column:
The columns used for HPLC are generally made up of stainless
steel . so they can withstand up to high pressure [8000psi]
Straight columns of 20-50 cm in length & 1-4mm in diameter are
used
Particle size should be for porous particles 20-40μm. For porous
micro particles it should be 3-10 μm
• Stationary phases used in column:
Alumina
Silica
Polyvinyl acetate beads
19](https://image.slidesharecdn.com/royalppthplc-181213071305/85/Royal-ppt-hplc-19-320.jpg)







![APPLICATIONS :
Purification of samples
Identification of compounds
Determination of impurities
Separation of mixture of samples
ex: carbohydrates
Determine the concentration of drug
Biopharmaceutical &pharmacokinetic studies
Drug stability studies
Qualitative & quantitative analysis
Environmental applications[analysing air & water pollutants]
28](https://image.slidesharecdn.com/royalppthplc-181213071305/85/Royal-ppt-hplc-28-320.jpg)
![QUALITATIVE ANALYSIS:
The identification of individual compounds in the sample;
The most common parameter for compound identification is its
retention time [is the amount of time a compound spends on the
column after injection]
Depending on the detector used, compound identification is also
based on the chemical structure, molecular weight or some other
molecular parameter.
30](https://image.slidesharecdn.com/royalppthplc-181213071305/85/Royal-ppt-hplc-30-320.jpg)

