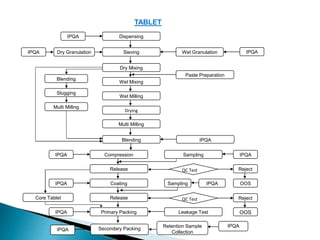
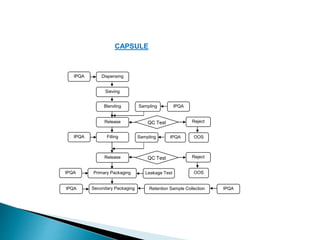
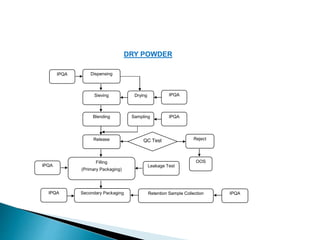
In-process controls (IPC) involve checks carried out during the manufacturing process to monitor and adjust the process if needed to ensure the product meets specifications. IPC includes testing in-process materials for identity, strength, quality and purity, as well as monitoring process parameters and environmental conditions. Written IPC procedures describe tests like tablet weight variation, disintegration time, and content uniformity. The objectives of IPC are quality control and process control. IPC results are documented and evaluated by quality control as part of release procedures.