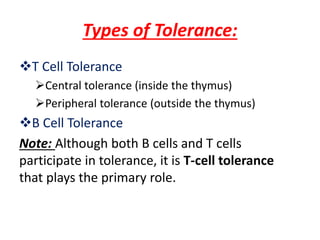
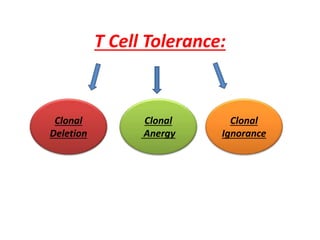
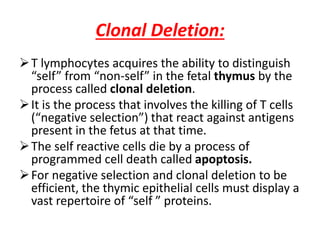
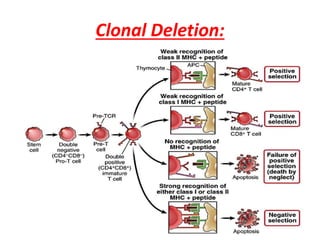
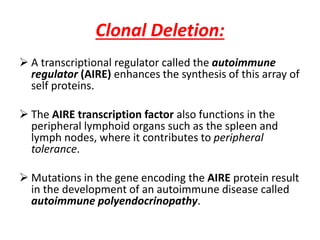
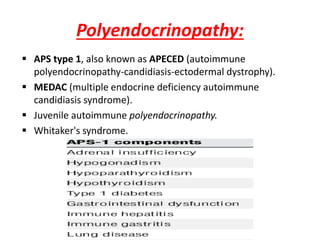
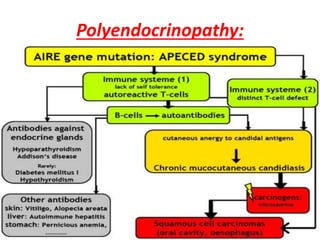
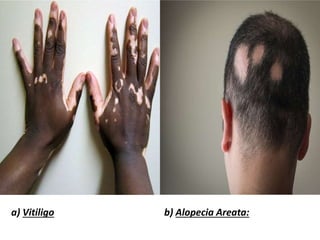
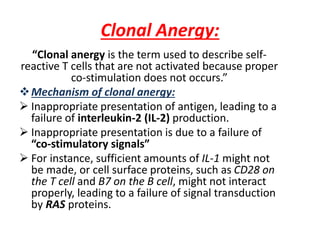
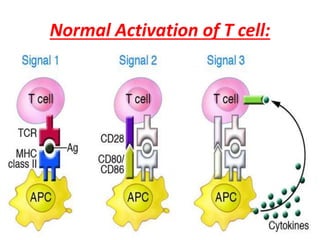
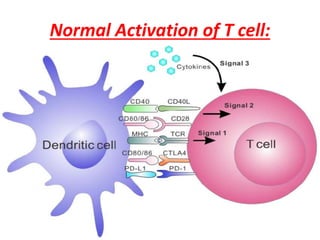
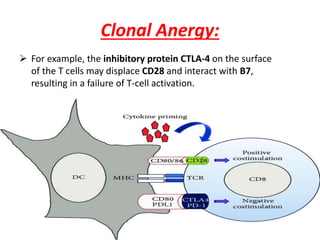
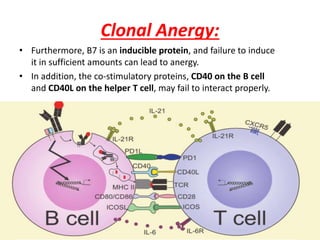
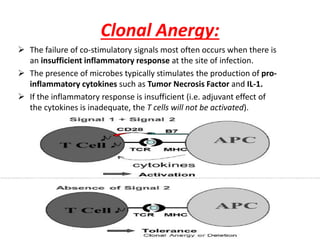
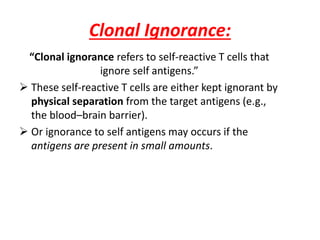
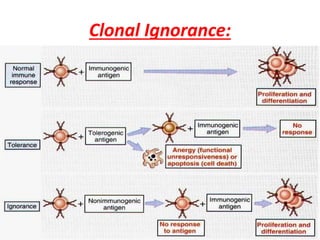
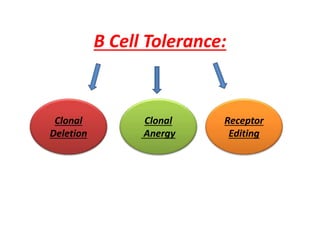
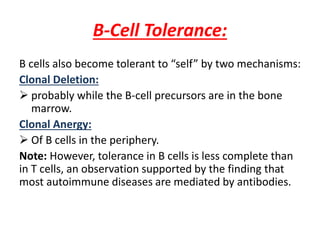
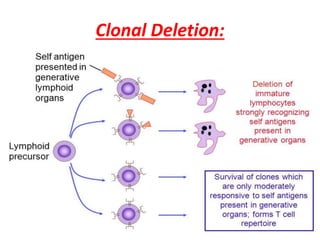
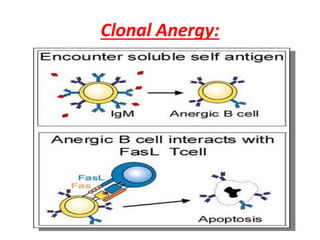
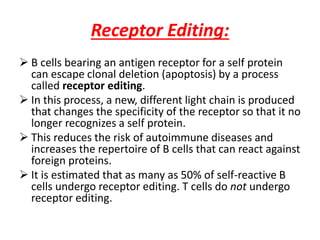
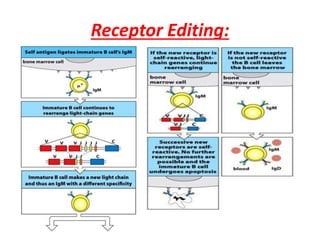
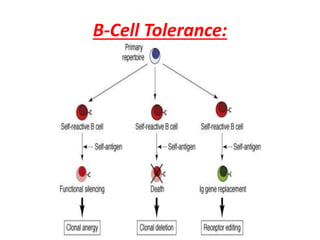
Central and peripheral tolerance mechanisms exist to prevent immune responses against self-antigens. Central tolerance occurs in the thymus through clonal deletion of self-reactive T cells. Peripheral tolerance can occur through clonal anergy, where T cells are not activated due to lack of co-stimulation, or clonal ignorance, where self-reactive T cells do not encounter self-antigens. B cell tolerance occurs through clonal deletion in the bone marrow or clonal anergy in the periphery.

![Tolerance:
“Tolerance is specific immunologic unresponsiveness
(i.e., an immune response to a certain antigen [or
epitope] does not occur, although the immune system is
otherwise functioning normally).”
In general, antigens that are present during embryonic
life are considered “self ” and do not stimulate an
immunologic response (i.e., we are tolerant to those
antigens).
The lack of an immune response in the fetus is caused
by the deletion of self-reactive T-cell precursors in the
thymus.](https://image.slidesharecdn.com/immunologicaltolerance-190424124046/85/Immunological-tolerance-2-320.jpg)