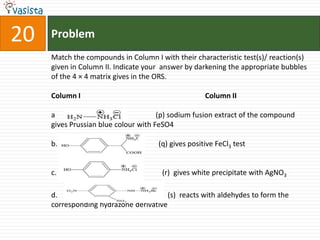
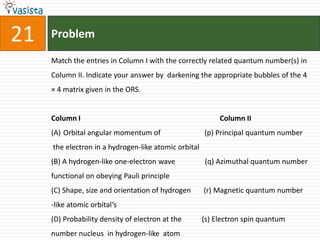
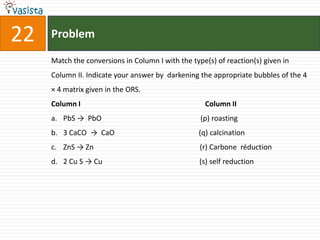
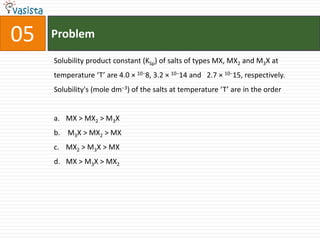
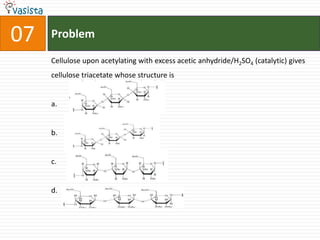
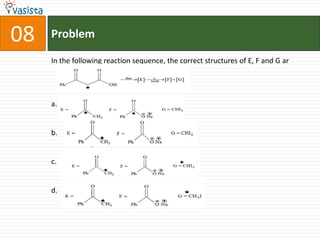
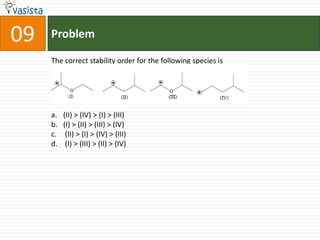
This document contains an unsolved chemistry practice paper from 2008 for IITJEE (Indian Institute of Technology Joint Entrance Examination). It has four sections testing different chemistry concepts through multiple choice questions. Section I has 9 objective questions testing concepts like IUPAC names, compound identification, hybridization, and solubility products. Section II has 4 reasoning questions requiring understanding of statements. Section III has 3 linked comprehension questions about reaction mechanisms. Section IV contains 3 matrix-match questions testing relationships between concepts.


![01ProblemThe IUPAC name of [Ni(NH3)4][NiCl4] isTetrachloronickel (II) – tetraamminenickel (II)Tetraamminenickel (II) – tetrachloronickel (II)Tetraamminenickel (II) – tetrachloronickelate (II)Tetrachloronickel (II) – tetraamminenickelate (0)](https://image.slidesharecdn.com/2008-ii-chemistry-110913053816-phpapp02/85/IIT-JEE-2008-ii-chemistry-3-320.jpg)
![Problem02Among the following the coloured compound isCuClK3[Cu(CN)4] CuF2 [Cu(CH3CN)4]BF4](https://image.slidesharecdn.com/2008-ii-chemistry-110913053816-phpapp02/85/IIT-JEE-2008-ii-chemistry-4-320.jpg)
![Problem03Both [Ni(CO)4] and [Ni(CN)4]2− are diamagnetic. The hybridization of nickel in these complexes, respectively, aresp3, sp3sp3, dsp2dsp2, sp3dsp2, dsp2](https://image.slidesharecdn.com/2008-ii-chemistry-110913053816-phpapp02/85/IIT-JEE-2008-ii-chemistry-5-320.jpg)
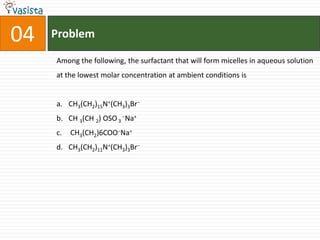


![Problem10STATEMENT-1: The geometrical isomers of the complex [M(NH3)4Cl2] are optically inactive.andSTATEMENT-2: Both geometrical isomers of the complex [M(NH3)4Cl2] possess axis of symmetry.STATEMENT-1 is True, STATEMENT-2 is True; STATEMENT-2 is correct explanation for STATEMENT-1STATEMENT-1 is True, STATEMENT-2 is True; STATEMENT-2 is NOT a correct explanation for STATEMENT-1STATEMENT-1 is True, STATEMENT-2 is FalseSTATEMENT-1 is False, STATEMENT-2 is TrueE](https://image.slidesharecdn.com/2008-ii-chemistry-110913053816-phpapp02/85/IIT-JEE-2008-ii-chemistry-13-320.jpg)
![Problem11STATEMENT-1: [Fe(H2O)5NO]SO4 is paramagnetic.andSTATEMENT-2: The Fe in [Fe(H2O)5NO]SO4 has three unpaired electrons.STATEMENT-1 is True, STATEMENT-2 is True; STATEMENT-2 is correct explanation for STATEMENT-1STATEMENT-1 is True, STATEMENT-2 is True; STATEMENT-2 is NOT a correct explanation for STATEMENT-1c. STATEMENT-1 is True, STATEMENT-2 is Falsed. STATEMENT-1 is False, STATEMENT-2 is True](https://image.slidesharecdn.com/2008-ii-chemistry-110913053816-phpapp02/85/IIT-JEE-2008-ii-chemistry-14-320.jpg)
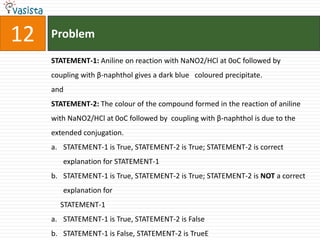



![Problem14Compound H is formed by the reaction of]a.b.c.d.](https://image.slidesharecdn.com/2008-ii-chemistry-110913053816-phpapp02/85/IIT-JEE-2008-ii-chemistry-19-320.jpg)