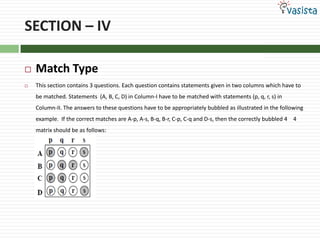
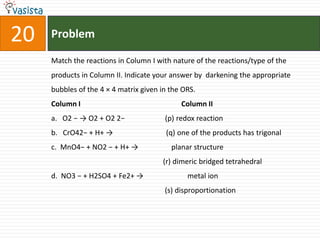
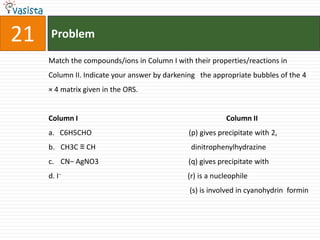
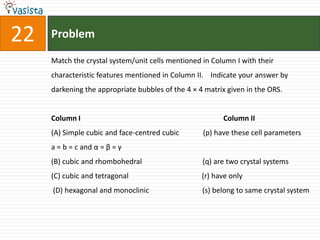
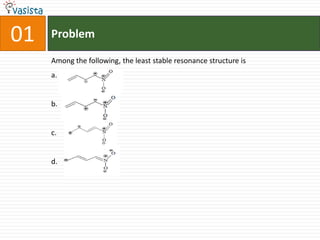
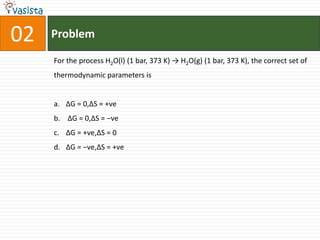
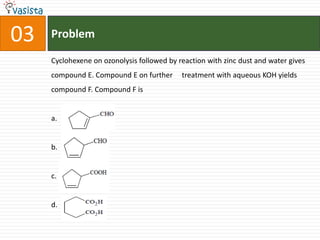
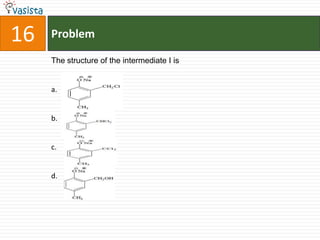
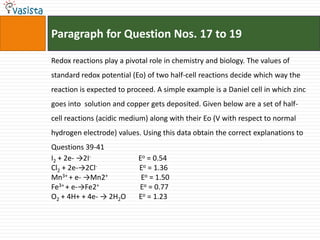
This document contains an unsolved chemistry paper from 2007 containing multiple choice and reason-type questions testing knowledge of chemistry concepts. The questions cover topics including resonance structures, thermodynamics, reaction kinetics, metal carbonyls, nuclear reactions, redox reactions, organic synthesis, and crystal systems. The document provides the questions and statements to be matched but does not include the answers. It is designed to test a test-taker's understanding of fundamental chemistry concepts through analysis of the problems presented.


![Problem05Among the following metal carbonyls, the C – O bond order is lowest in[Mn(CO)6]+ [Fe(CO)5][Cr(CO)6] [V(CO)6]-](https://image.slidesharecdn.com/2007-ii-chemistry-110913052131-phpapp02/85/IIT-JEE-2007-ii-chemistry-7-320.jpg)


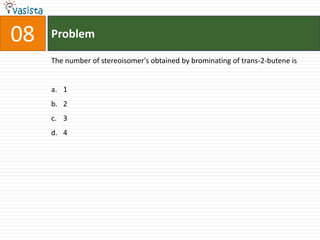
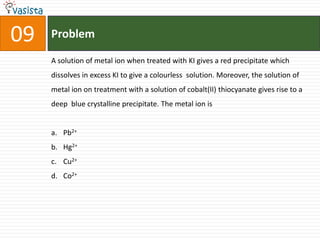


![Problem11STATEMENT-1: Alkali metals dissolves in liquid ammonia to give blue solutionbecauseSTATEMENT-2: Alkali metals in liquid ammonia give solvated species of the type [M(NH3)n]+ (M = alkali metals).Statement-1 is True, Statement-2 is True; Statement-2 is a correct explanation for Statement-1Statement-1 is True, Statement-2 is True; Statement-2 is NOT a correct explanation for Statement-1Statement-1 is True, Statement-2 is FalseStatement-1 is False, Statement-2 is True](https://image.slidesharecdn.com/2007-ii-chemistry-110913052131-phpapp02/85/IIT-JEE-2007-ii-chemistry-14-320.jpg)
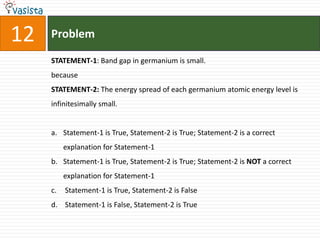






![Problem19Sodium fusion extract, obtained from aniline, on treatment with iron (II) sulphate and H2SO4 in presence of air gives a Prussian blue precipitate. The blue colour is due to the formation ofFe4[Fe(CN)6]3Fe3[Fe(CN)6]2Fe4[Fe(CN)6]2Fe3[Fe(CN)6]3](https://image.slidesharecdn.com/2007-ii-chemistry-110913052131-phpapp02/85/IIT-JEE-2007-ii-chemistry-25-320.jpg)