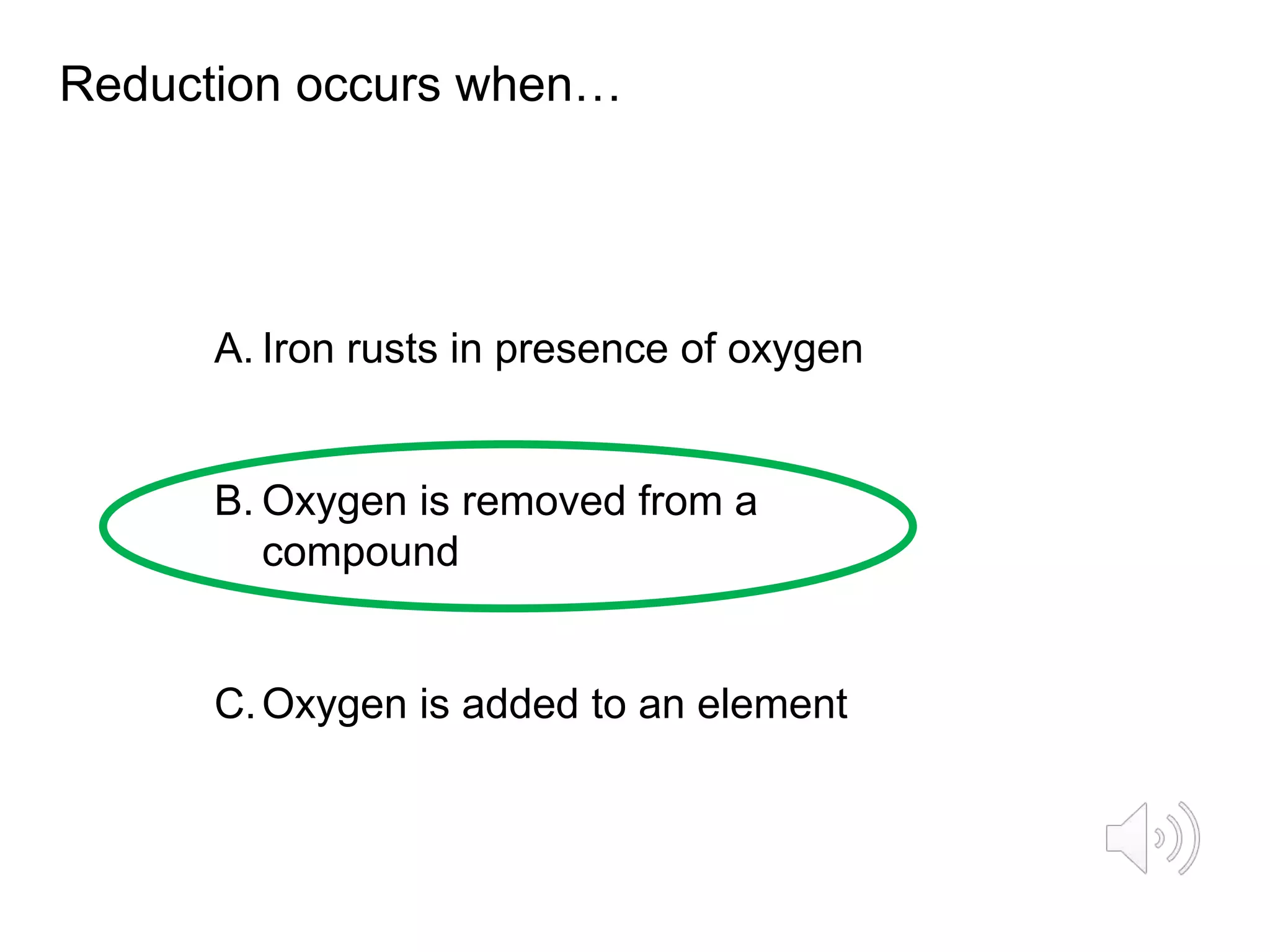
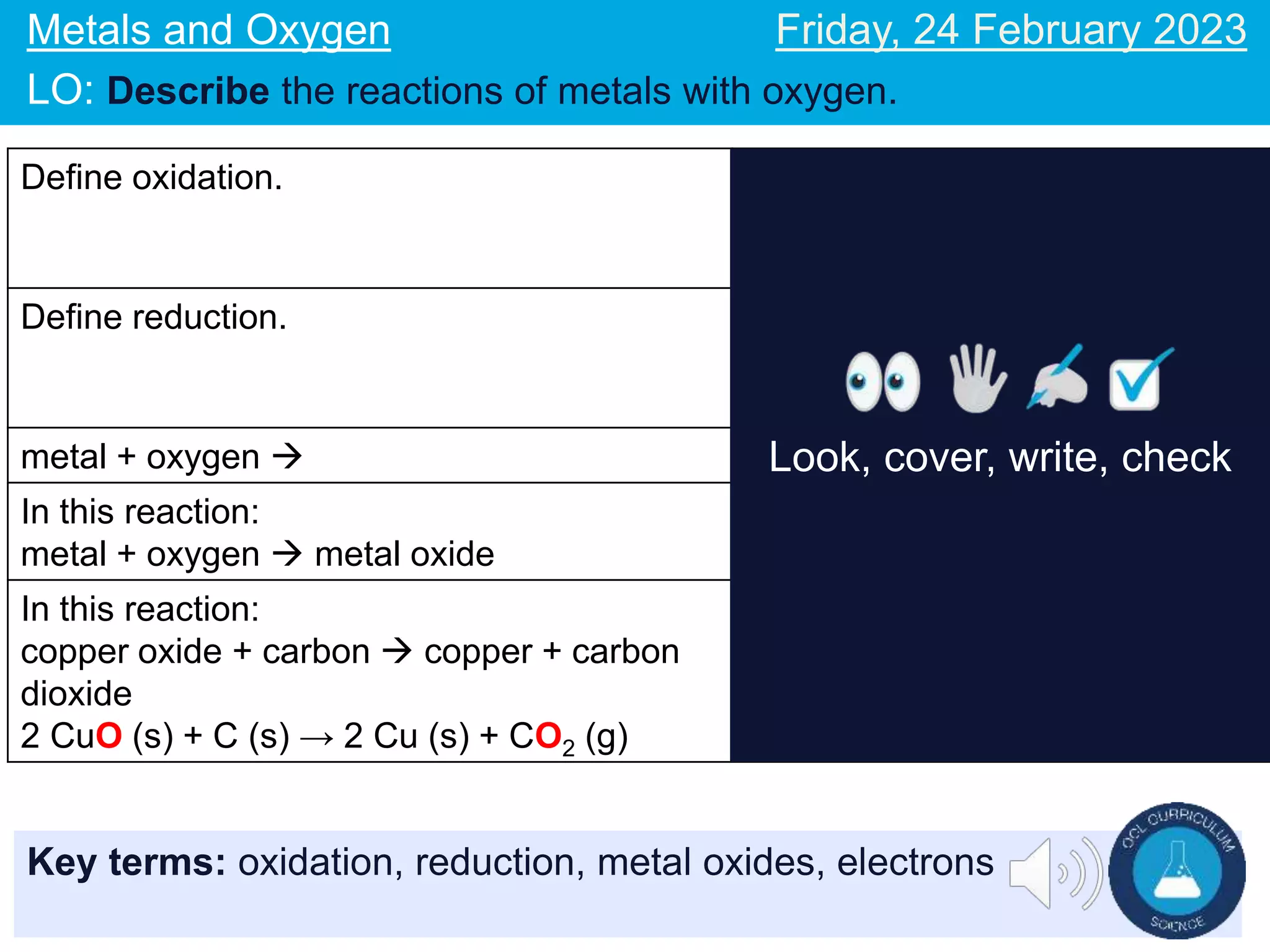
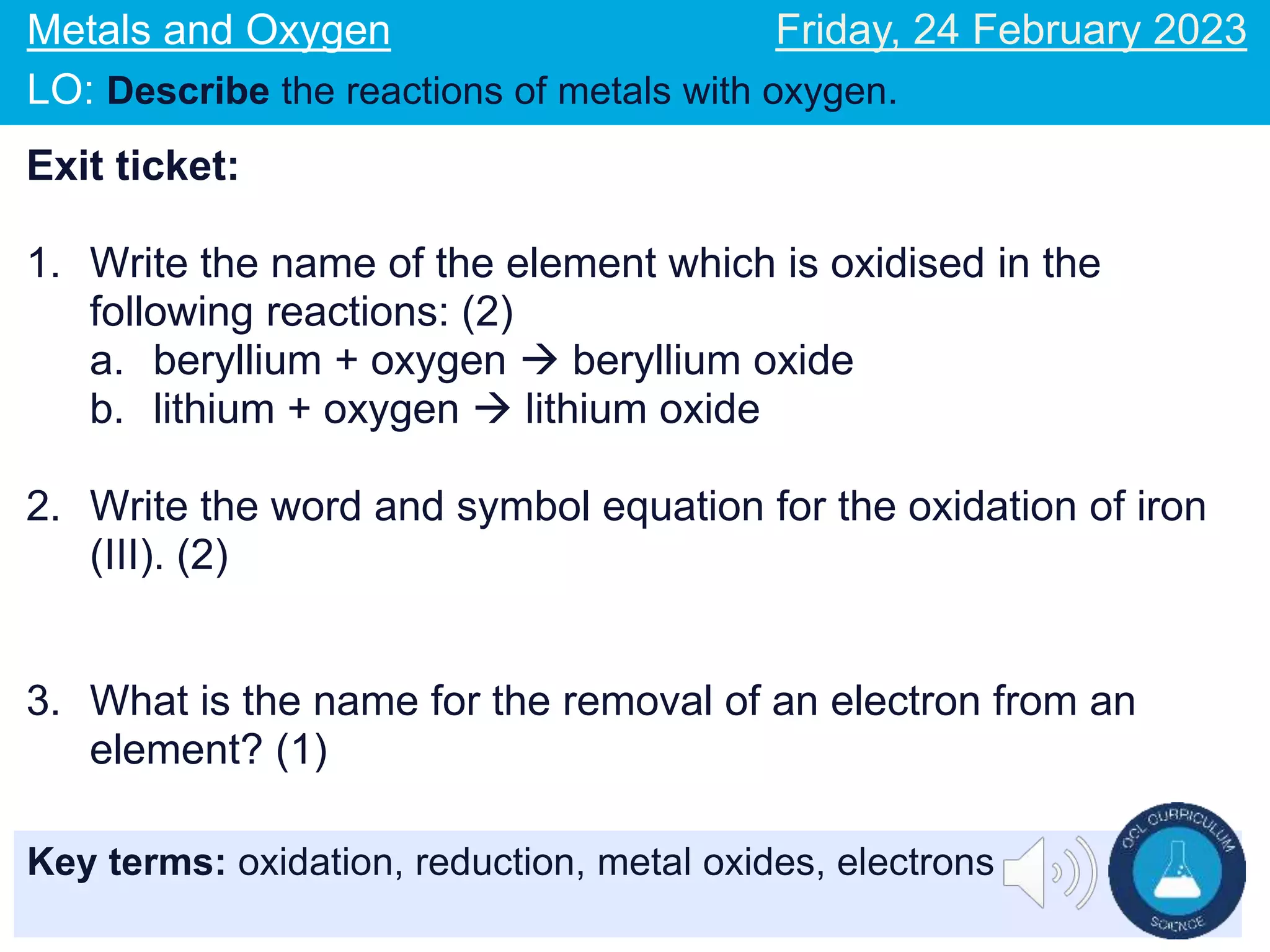
The document describes reactions of metals with oxygen. It defines oxidation as the addition of oxygen to an element and the loss of electrons, and reduction as the removal of oxygen from an element and the gain of electrons. Word and symbol equations are provided for reactions of various metals like iron and magnesium with oxygen, where the metal gains oxygen and is oxidized to form metal oxides. Examples of oxidation and reduction reactions are discussed along with related concepts like electrons lost or gained in the processes.