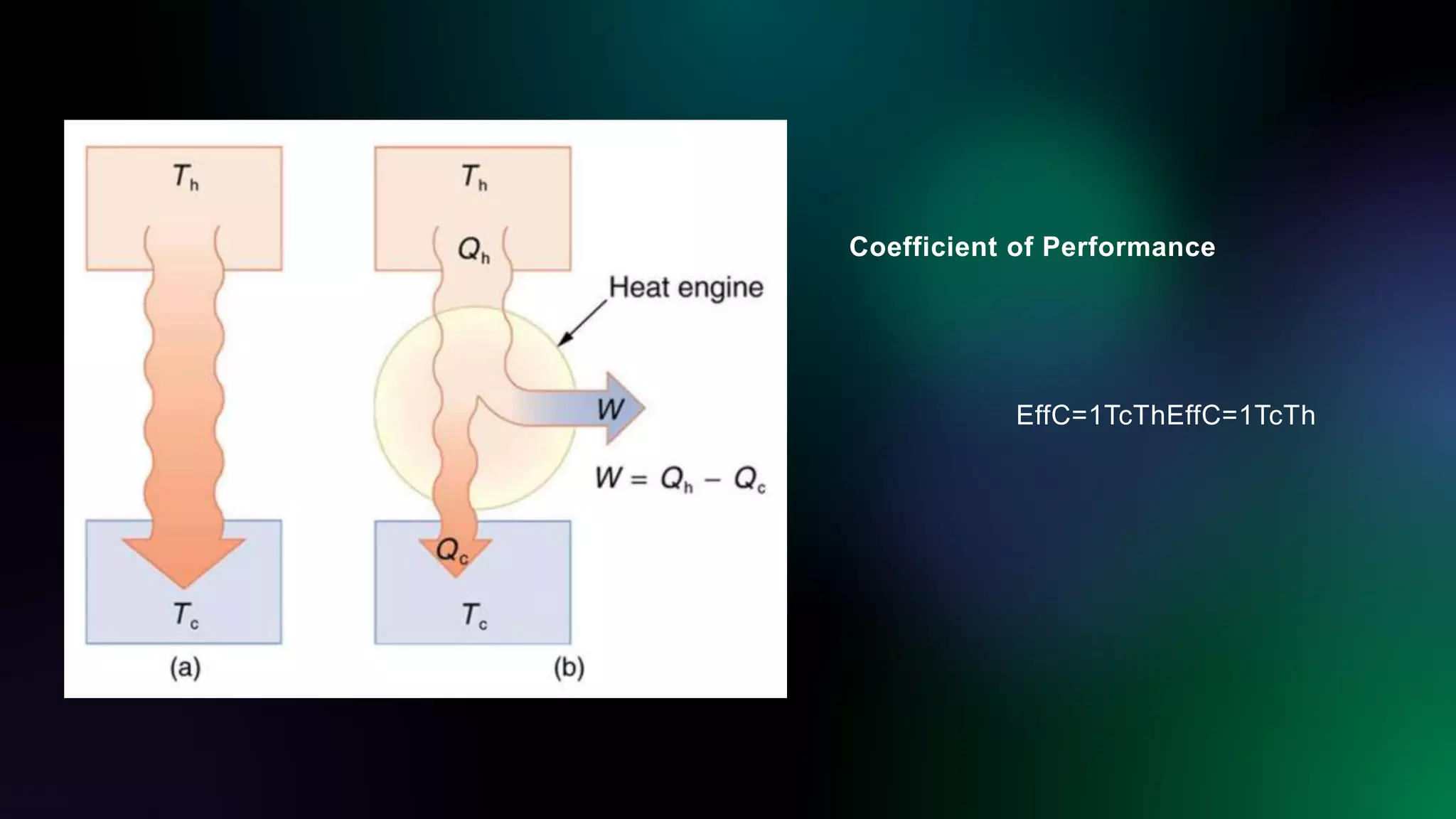
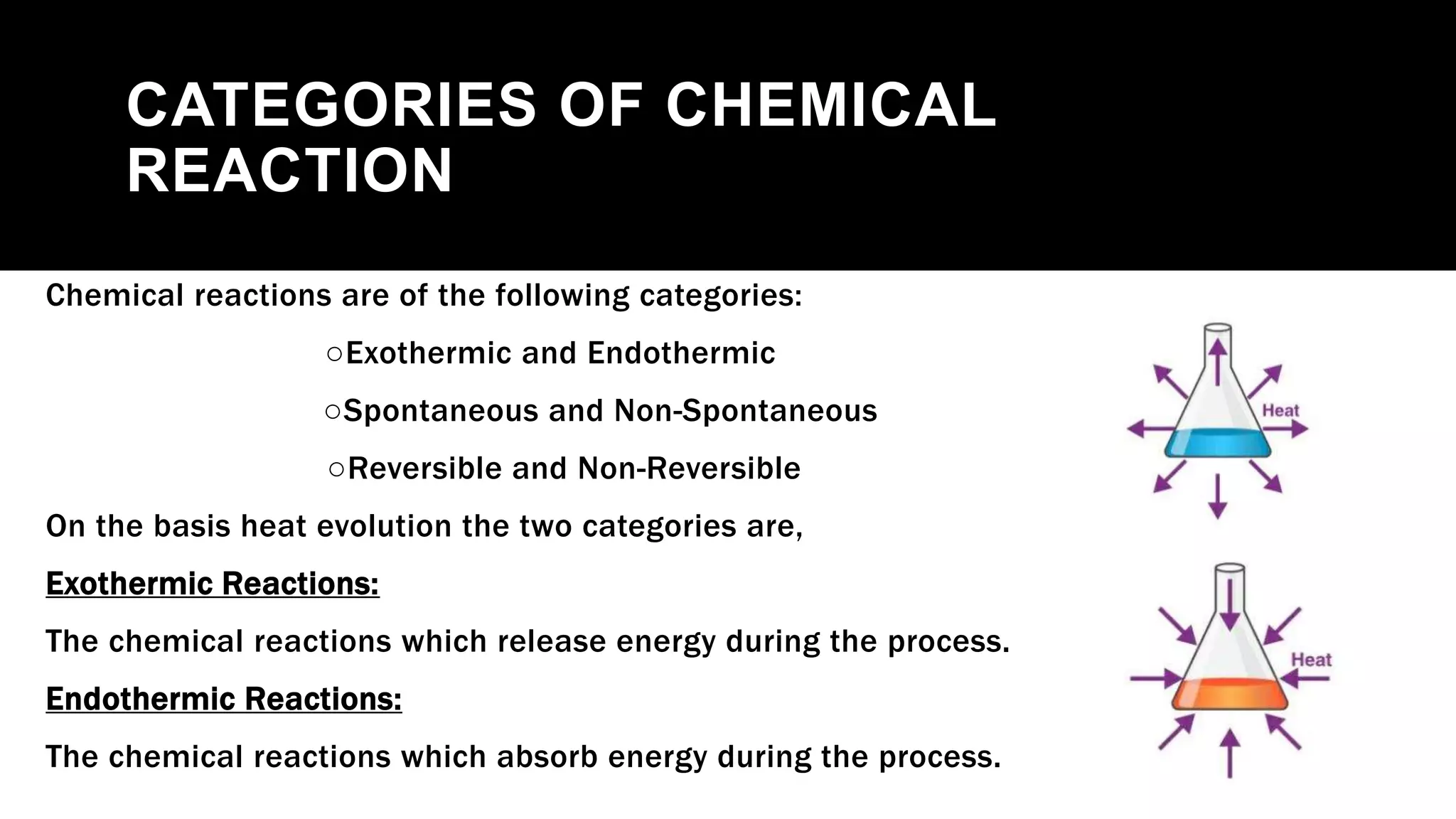
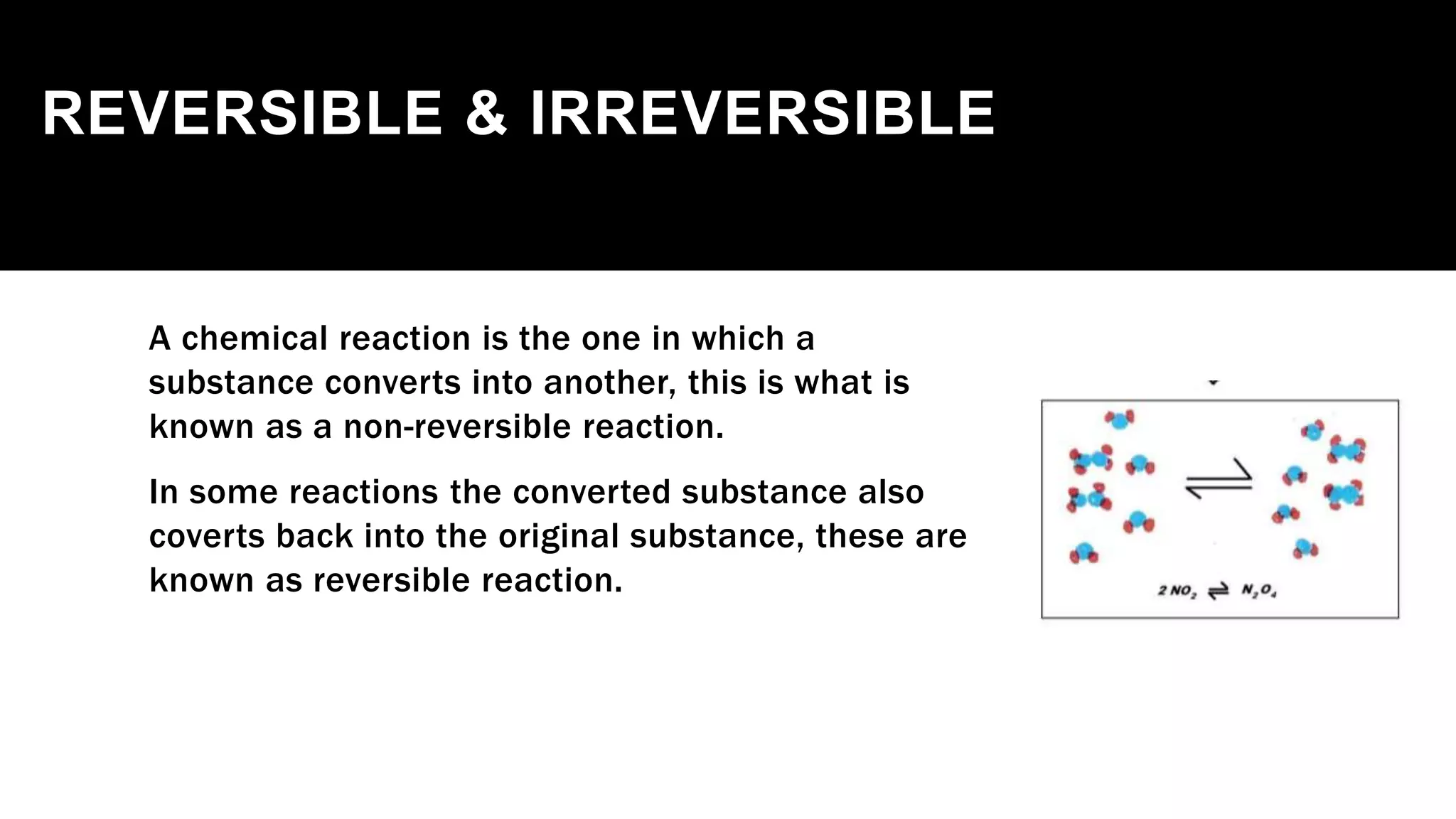
The document outlines the three laws of thermodynamics, explaining concepts such as energy conservation, entropy, and temperature relationships with entropy in crystals. It further categorizes chemical reactions into exothermic, endothermic, spontaneous, and non-spontaneous types, detailing how Gibbs energy influences their spontaneity. The document concludes with a discussion on reversible and non-reversible reactions, emphasizing the impact of temperature on the equilibrium state and thermochemistry.