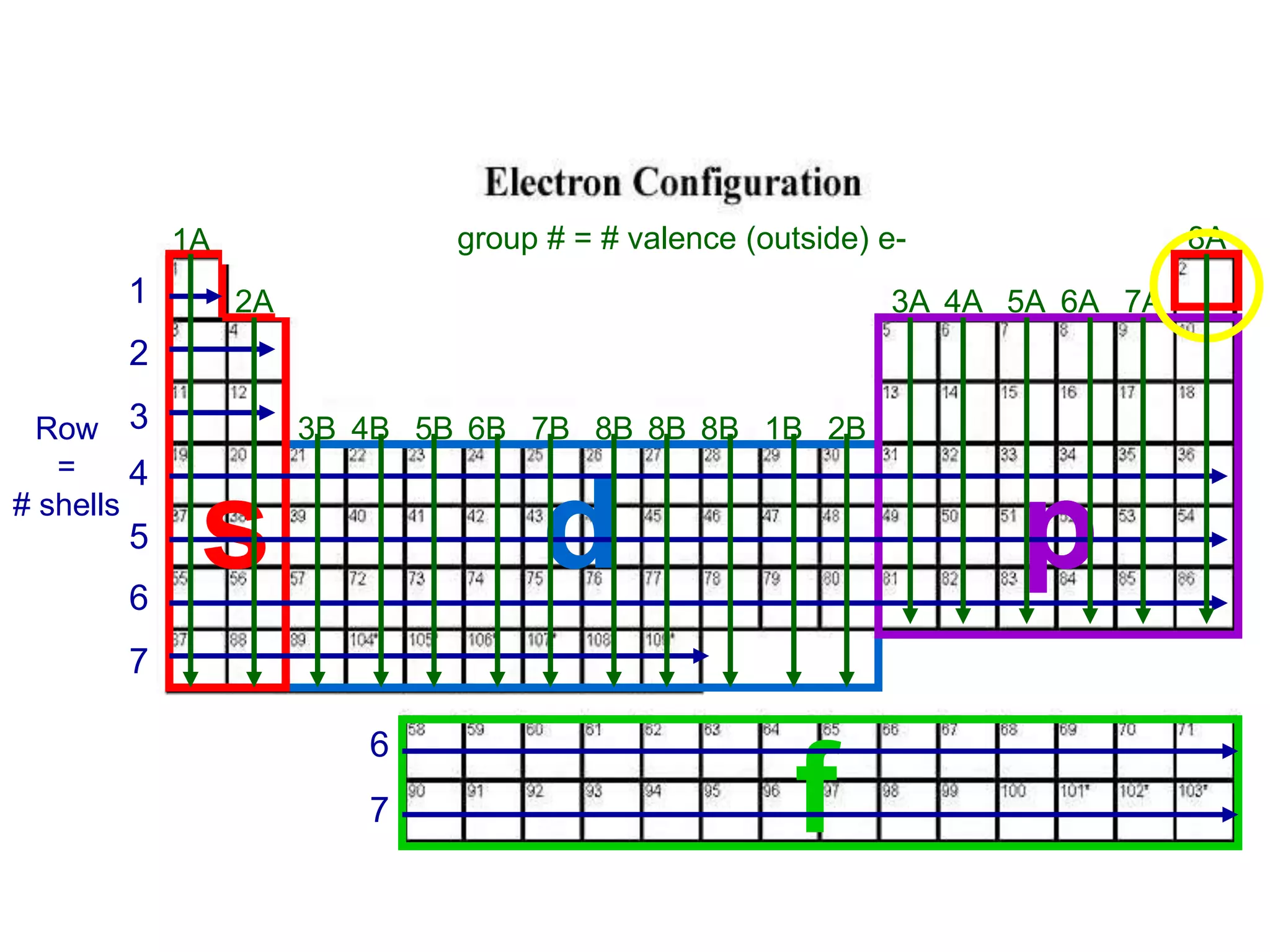
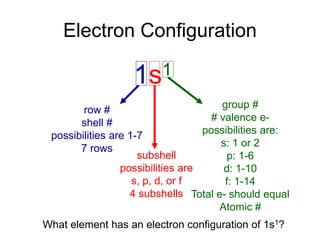
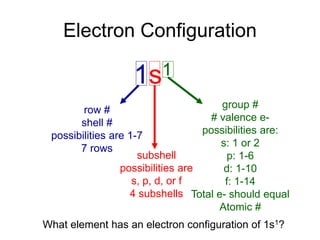
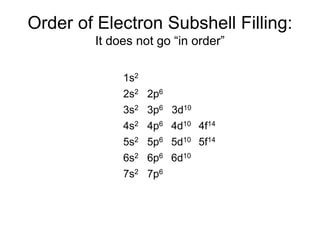
1) The document discusses electron configurations and the order in which atomic orbitals are filled. It provides guidance on asking questions to determine the electron configuration for any element, including the period, number of shells, group, valence electrons, and occupied subshells.
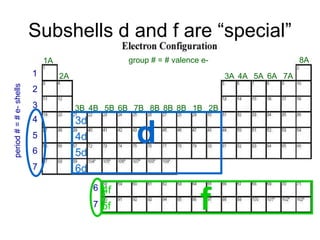
2) The d and f subshells are described as "special" because they fill in a different order than other subshells and have unique occupation ranges from 1-10 electrons for d and 1-14 for f.
3) The order of filling for different subshells is specified, with s and p orbitals filling first before the d and f orbitals are occupied.