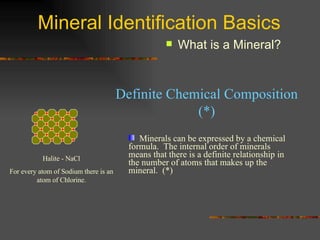
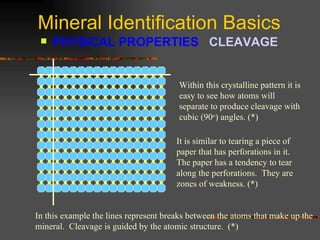
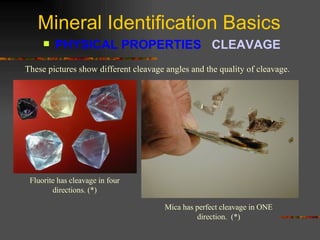
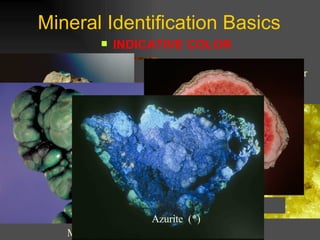
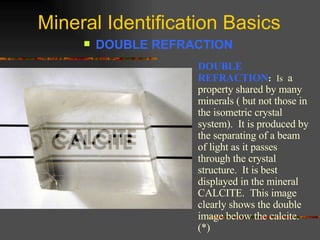
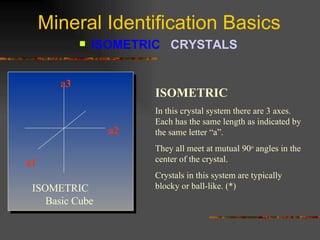
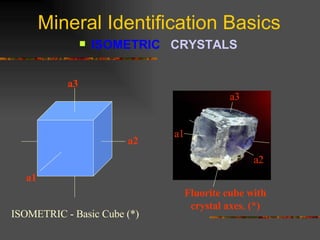
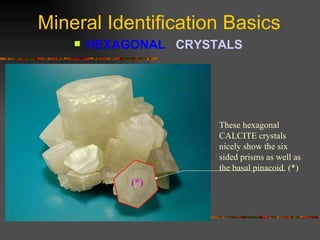
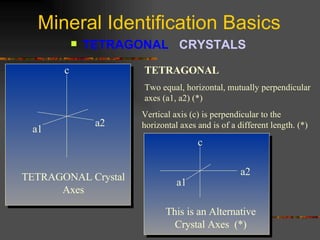
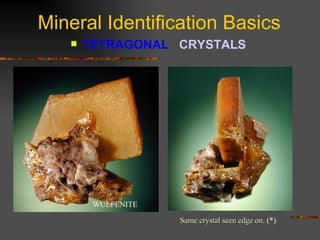
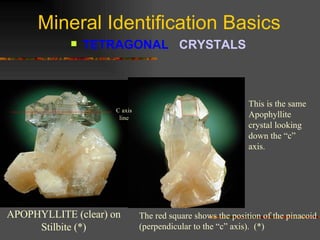
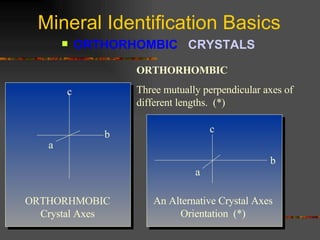
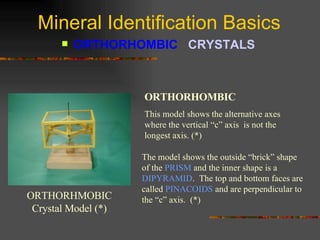
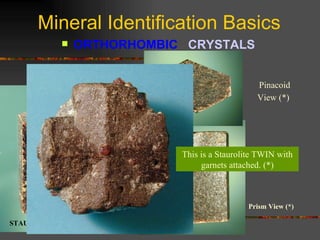
The document provides an overview of the key concepts for identifying minerals, including their physical properties. It discusses that minerals must be naturally occurring, inorganic, have a definite crystalline structure, and chemical composition. It then covers several important physical properties for identification, including hardness based on Mohs scale, cleavage, fracture, streak (color as a powder), and luster (quality of reflected light). Examples are given for each property to illustrate how they can be used to identify unknown minerals. The document aims to teach the fundamental characteristics and tests used in mineral identification.