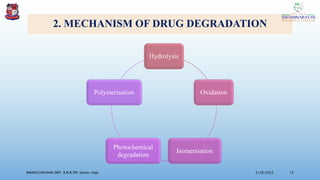
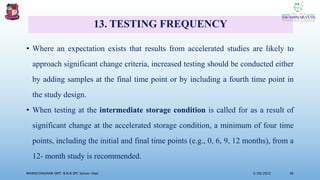
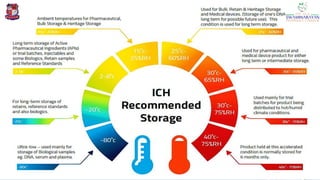
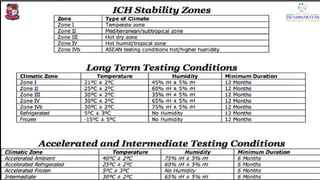
The document discusses the ICH guidelines for the stability testing of drug substances and products, outlining various types of stability including physical, chemical, and microbiological. It details the mechanisms of drug degradation, the importance of stability studies, and the specific testing requirements for registration applications across different regions. Key principles include establishing shelf life, testing conditions, and addressing environmental factors affecting drug stability.